Label free electrochemical DNA biosensor for COVID-19 diagnosis
- PMID: 36228554
- PMCID: PMC9546783
- DOI: 10.1016/j.talanta.2022.123992
Label free electrochemical DNA biosensor for COVID-19 diagnosis
Abstract
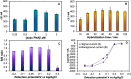
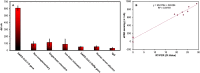
The COVID-19 pandemic has significantly increased the development of the development of point-of-care (POC) diagnostic tools because they can serve as useful tools for detecting and controlling spread of the disease. Most current methods require sophisticated laboratory instruments and specialists to provide reliable, cost-effective, specific, and sensitive POC testing for COVID-19 diagnosis. Here, a smartphone-assisted Sensit Smart potentiostat (PalmSens) was integrated with a paper-based electrochemical sensor to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A disposable paper-based device was fabricated, and the working electrode directly modified with a pyrrolidinyl peptide nucleic acid (acpcPNA) as the biological recognition element to capture the target complementary DNA (cDNA). In the presence of the target cDNA, hybridization with acpcPNA probe blocks the redox conversion of a redox reporter, leading to a decrease in electrochemical response correlating to SARS-CoV-2 concentration. Under optimal conditions, a linear range from 0.1 to 200 nM and a detection limit of 1.0 pM were obtained. The PNA-based electrochemical paper-based analytical device (PNA-based ePAD) offers high specificity toward SARS-CoV-2 N gene because of the highly selective PNA-DNA binding. The developed sensor was used for amplification-free SARS-CoV-2 detection in 10 nasopharyngeal swab samples (7 SARS-CoV-2 positive and 3 SARS-CoV-2 negative), giving a 100% agreement result with RT-PCR.
Keywords: COVID-19; PNA; Paper-based device; Point-of-care testing; Portable potentiostat; SARS-CoV-2.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- World Health Organization (WHO) 2022. Weekly Epidemiological Update on COVID-19 - 25 January 2022.https://www.who.int/publications/m/item/weekly-epidemiological-update-on... (accessed January 28, 2022)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

