Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial
- PMID: 36228641
- PMCID: PMC9635892
- DOI: 10.1016/S2213-2600(22)00298-3
Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial
Abstract
Background: COVID-19 disease is accompanied by a dysregulated immune response and hypercoagulability. The Anti-Coronavirus Therapies (ACT) inpatient trial aimed to evaluate anti-inflammatory therapy with colchicine and antithrombotic therapy with the combination of rivaroxaban and aspirin for prevention of disease progression in patients hospitalised with COVID-19.
Methods: The ACT inpatient, open-label, 2 × 2 factorial, randomised, controlled trial was done at 62 clinical centres in 11 countries. Patients aged at least 18 years with symptomatic, laboratory confirmed COVID-19 who were within 72 h of hospitalisation or worsening clinically if already hospitalised were randomly assigned (1:1) to receive colchicine 1·2 mg followed by 0·6 mg 2 h later and then 0·6 mg twice daily for 28 days versus usual care; and in a second (1:1) randomisation, to the combination of rivaroxaban 2·5 mg twice daily plus aspirin 100 mg once daily for 28 days versus usual care. Investigators and patients were not masked to treatment allocation. The primary outcome, assessed at 45 days in the intention-to-treat population, for the colchicine randomisation was the composite of the need for high-flow oxygen, mechanical ventilation, or death; and for the rivaroxaban plus aspirin randomisation was the composite of major thrombosis (myocardial infarction, stroke, acute limb ischaemia, or pulmonary embolism), the need for high-flow oxygen, mechanical ventilation, or death. The trial is registered at www.
Clinicaltrials: gov, NCT04324463 and is ongoing.
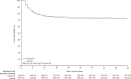
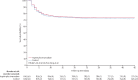
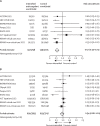
Findings: Between Oct 2, 2020, and Feb 10, 2022, at 62 sites in 11 countries, 2749 patients were randomly assigned to colchicine or control and the combination of rivaroxaban and aspirin or to the control. 2611 patients were included in the analysis of colchicine (n=1304) versus control (n=1307); 2119 patients were included in the analysis of rivaroxaban and aspirin (n=1063) versus control (n=1056). Follow-up was more than 98% complete. Overall, 368 (28·2%) of 1304 patients allocated to colchicine and 356 (27·2%) of 1307 allocated to control had a primary outcome (hazard ratio [HR] 1·04, 95% CI 0·90-1·21, p=0·58); and 281 (26·4%) of 1063 patients allocated to the combination of rivaroxaban and aspirin and 300 (28·4%) of 1056 allocated to control had a primary outcome (HR 0·92, 95% CI 0·78-1·09, p=0·32). Results were consistent in subgroups defined by vaccination status, disease severity at baseline, and timing of randomisation in relation to onset of symptoms. There was no increase in the number of patients who had at least one serious adverse event for colchicine versus control groups (87 [6·7%] of 1304 vs 90 [6·9%] of 1307) or with rivaroxaban and aspirin versus control groups (85 [8·0%] vs 91 [8·6%]). Among patients assigned to colchicine, 8 (0·61%) had adverse events that led to discontinuation of study drug, mostly gastrointestinal in nature. 17 (1·6%) patients assigned to the combination of rivaroxaban and aspirin had bleeding compared with seven (0·66%) of those allocated to control (p=0·042); the number of serious bleeding events was two (0·19%) versus six (0·57%), respectively (p=0·18). No patients assigned to rivaroxaban and aspirin had serious adverse events that led to discontinuation of study drug.
Interpretation: Among patients hospitalised with COVID-19, neither colchicine nor the combination of rivaroxaban and aspirin prevent disease progression or death.
Funding: Canadian Institutes for Health Research, Bayer, Population Health Research Institute, Hamilton Health Sciences Research Institute, Thistledown Foundation.
Translations: For the Portuguese, Russian and Spanish translations of the abstract see Supplementary Materials section.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JWE reports grant or in-kind support from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Pfizer, Janssen, Sanofi-Aventis and honoraria from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Daiichi-Sankyo, Eli-Lilly, Glaxo-Smith-Kline, Merck, Pfizer, Janssen, Sanofi-Aventis, Servier. SSJ reports grant support from Boston Scientific, honoraria from Medtronic, Penumbra. EPB-C reports grant support from Bayer, Roche, BMS-Pfizer. RPW reports grant support from Bayer, Roche, BMS-Pfizer, grant and honorarium from Boehringer-Ingelheim, and consultancy fees from Atricure and Phasebio. MLD reports grant support from the Population Health Research Institute (PHRI) to manage the ACT study in Argentina. RD reports grant support from PHRI to manage the ACT study in Argentina. AA reports institutional grant support from Bayer and EMS, and lecture fees from Bayer and Sanofi-Aventis. SW reports grant support from NIH, honoraria from Pfizer, and safety monitoring committee of an AIDS Clinical Trial Group. RDL reports institutional grant support from Bristol Myers Squibb, Glaxo Smith Kline, Medtronic, Pfizer, and Sanofi, consulting fees from Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, Sanofi, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck, and Portola, honoraria from Pfizer and meeting travel support from IQVIA. OB reports grant support from Astra Zeneca, Bayer, Amgen, Novartis, Servier, Pfizer. SSA reports grants from CIHR and PHAC, honoraria and consulting fees from Bayer and Janssen Pharma, meeting support from Heart and Stroke Canada, is committee member at the American Heart Association, the Canadian Cardiovascular Society, and Heart and Stroke Canada. JB reports consulting fees from Bayer. SC reports ACT study funding from Bayer, owns Bayer stock, is a board member of Canadian Arrhythmia Network, is an institutional advisory board member at the Institute of Circulatory and Respiratory Health, Canadian Institutes for Health Research. MEF reports institutional grants from Amgen, Astra Zeneca, Novartis, and Novo Nordisk and consulting fees from Otitopic. ML reports participation in vaccine advisory boards for Medicago, Pfizer, and Sanofi and is on the data safety and monitoring board for CanSino Biologics. SY reports institutional grant support from Bayer and honoraria, and travel costs for lectures from Bayer. SR, LX, LH, SIB, AY, SKS, WMT, MH, WH, AA, OD, CF, and PL-J have no disclosures to report.
Figures

Comment in
-
COVID-19: ACT trials for colchicine and antithrombotic therapies.Lancet Respir Med. 2022 Dec;10(12):1106-1108. doi: 10.1016/S2213-2600(22)00368-X. Epub 2022 Oct 10. Lancet Respir Med. 2022. PMID: 36228642 Free PMC article. No abstract available.
References
-
- Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. - PubMed
-
- Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. - PubMed
-
- Bollyky TJ, Nuzzo J, Huhn N, Kiernan S, Pond E. Global vaccination must be swifter. Nature. 2022;603:788–792. - PubMed
Uncited References
-
- Wang JH, Chen RD, Yang HK, et al. Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19. J Med Virol. 2021;93:2908–2917. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

