UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial
- PMID: 36228643
- PMCID: PMC11575699
- DOI: 10.1016/S2352-3026(22)00245-9
UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial
Abstract
Background: The prognosis for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia remains poor. UCART19, an allogeneic genome-edited anti-CD19 chimeric antigen receptor (CAR) T-cell product derived from healthy donors and available for immediate clinical use, offers a potential therapeutic option for such patients. The CALM trial is a first-in-human study evaluating the safety and antileukaemic activity of UCART19 in adult patients with relapsed or refractory B-cell acute lymphoblastic leukaemia.
Methods: This phase 1, open-label study was conducted at eight centres across France, the UK, the USA, and Japan. Adult patients aged 16-70 years with CD19-positive relapsed or refractory B-cell acute lymphoblastic leukaemia who had morphological relapse or a minimal residual disease level of at least 1 × 10-3 and had exhausted standard treatment options were enrolled in the study, which comprised a dose-escalation phase of up to three UCART19 doses followed by a safety expansion phase. Patients underwent lymphodepletion with fludarabine (30 mg/m2 per day intravenously for 3 days) and cyclophosphamide (500 mg/m2 per day intravenously for 3 days) with or without alemtuzumab (1 mg/kg or 40 mg or 60 mg over 5 days) and received UCART19 doses of 6 × 106, 6-8 × 107, or 1·8-2·4 × 108 total CAR T cells intravenously, followed by safety evaluation and disease response assessments. The primary endpoint was incidence and severity of adverse events. Secondary endpoints were the overall response rate, duration of response, relapse-free survival, progression-free survival, and overall survival. This trial is registered with ClinicalTrials.gov (NCT02746952) and is complete.
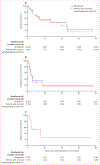
Findings: Between Aug 1, 2016, and June 30, 2020, 25 patients were enrolled in the study and treated with UCART19. Median duration of follow-up was 12·8 months (IQR 2·8-24·8). Median age was 37 years (IQR 28-45). 14 (56%) patients were male and 11 (44%) female. 17 (68%) patients were White, two (8%) Black, two (8%) Asian, and four (16%) from other racial or ethnic groups. Three patients developed dose-limiting toxicities (one at each dose level); one had grade 4 cytokine release syndrome and two had grade 4 prolonged cytopenias. Grade 3 or higher cytokine release syndrome was reported in six (24%) patients and grade 3 or higher neurological toxicity in one (4%) patient. Grade 3 or higher infections occurred in seven (28%) patients, and grade 4 prolonged cytopenia in four (16%) patients. Two (8%) patients developed grade 1 acute cutaneous graft-versus-host disease. 14 patients died, nine from progressive disease and five from infections or other complications, of which four were considered to be related to UCART19 or lymphodepletion, or both. After a median of follow-up of 12·8 months (IQR 2·8-24·8), overall response rate was 48% (95% CI 28-69; 12 of 25 patients), duration of response and median relapse-free survival were 7·4 months (95% CI 1·8 to not calculable), progression-free survival was 2·1 months (95% CI 1·2-2·8), and overall survival was 13·4 months (95% CI 4·8-23·0).
Interpretation: UCART19 had a manageable safety profile, and showed evidence of antileukaemic activity in heavily pretreated adult patients with relapsed or refractory B-cell acute lymphoblastic leukaemia. This study shows that allogeneic off-the-shelf CAR T cells can be used safely to treat patients with relapsed B-cell acute lymphoblastic leukaemia.
Funding: Servier.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests RB received research funding from Servier and Allogene and has participated in advisory boards for Kite/Gilead, Novartis, Celgene/Bristol-Myers Squibb, Cellectis, and Enara Bio. NJ reports grants and personal fees from Servier during the conduct of the study; grants, personal fees, and non-financial support from Pharmacyclics, AstraZeneca, Genentech, Verastem, Pfizer, AbbVie, ADC Therapeutics, Precision Biosciences, and Adaptive Biotechnologies; personal fees and non-financial support from Janssen; and grants and non-financial support from Bristol-Myers Squib, Celgene, Seattle Genetics, Incyte, and Cellectis, outside the submitted work. MVM is an inventor on patents related to adoptive cell therapies held by Massachusetts General Hospital and the University of Pennysylvania (some of which are licensed to Novartis), holds equity in TCR2 and Century Therapeutics, and has served as a consultant for multiple companies involved in cell therapies. NB, CG, and AJ received research funding from Servier. DY reports grants and non-financial support from Servier during the conduct of the study, and non-financial support from Amgen and personal fees from Pfizer, outside the submitted work. MK reports grants and other from AbbVie, F. Hoffman La-Roche, Stemline Therapeutics, Forty-Seven, and Genentech; grants from Eli Lilly, Cellectis, Calithera, Ablynx, Agios, Ascentage, Astra Zeneca, Rafael Pharmaceutical, and Sanofi; and honoraria from Reata Pharmaceutical and Janssen outside the submitted work. MK also has a patent US 7,795,305 B2 “CDDO-compounds and combination therapies thereof” with royalties paid to Reata Pharm, a patent “Combination therapy with a mutant IDH1 inhibitor and a BCL-2” licensed to Eli Lilly, and a patent 62/993,166 “Combination of a MCL-1 inhibitor and midostaurin, uses and pharmaceutical compositions thereof” pending to Novartis. MJF has advisory roles with Kite/Gilead, Novartis, Celgene/Bristol-Myers Squibb, Arcellx, and Iovance, and recieves trial support from Kite/Gilead and Novartis. TT reports personal fees from Merck Sharp & Dohme; grants and personal fees from Kyowa Kirin; personal fees from Takeda, Pfizer, and Bristol-Myers Squibb; grants from Chugai, Sanofi, Astellas, Teijin Pharma, Fuji Pharma, Nippon Shinyaku, Japan Society for the Promotion of Science KAKENHI (17H04206), and The Center of Innovation Program from Japan Science and Technology Agency; non-financial support from Janssen; and grants, personal fees, and non-financial support from Novartis, outside the submitted work. KK reports grants and personal fees from AbbVie, Chugai, Eisai, Janssen, Novartis, Daiichi Sankyo, Takeda, and Kyowa-Kirin, and personal fees from AstraZeneca, Celgene, Ono, MSD, Mundi, Dainippon-Sumitomo, and Bristol-Myers Squibb, outside the submitted work. FBo, FBi, IM, SD, MA-C, MP, and SF are employees of Servier. SB and AG-B were previous employees of Servier. EB reports personal fees from Novartis, Astellas, Alexion, Jazz Pharmaceuticals, and Gilead outside the submitted work. MM reports grants and personal fees from Sanofi and Jazz Pharmaceuticals; personal fees from Janssen, Celgene, Bristol-Myers Squibb, Takeda, and Amgen; and grants from Roche, outside the submitted work.
Figures


Comment in
-
Allogeneic CAR T cells show promise.Nat Rev Clin Oncol. 2022 Dec;19(12):748. doi: 10.1038/s41571-022-00703-4. Nat Rev Clin Oncol. 2022. PMID: 36271140 No abstract available.
References
-
- Food US and Administration Drug. FDA approval brings first gene therapy to the United States. Aug 30, 2017. https://www.fda.gov/news-events/press-announcements/fda-approval-brings-... (accessed Jan 28, 2022).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

