Monogenic early-onset lymphoproliferation and autoimmunity: Natural history of STAT3 gain-of-function syndrome
- PMID: 36228738
- PMCID: PMC10081938
- DOI: 10.1016/j.jaci.2022.09.002
Monogenic early-onset lymphoproliferation and autoimmunity: Natural history of STAT3 gain-of-function syndrome
Erratum in
-
Corrigendum.J Allergy Clin Immunol. 2024 Apr;153(4):1167. doi: 10.1016/j.jaci.2024.02.001. J Allergy Clin Immunol. 2024. PMID: 38582550 No abstract available.
Abstract
Background: In 2014, germline signal transducer and activator of transcription (STAT) 3 gain-of-function (GOF) mutations were first described to cause a novel multisystem disease of early-onset lymphoproliferation and autoimmunity.
Objective: This pivotal cohort study defines the scope, natural history, treatment, and overall survival of a large global cohort of patients with pathogenic STAT3 GOF variants.
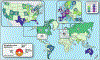
Methods: We identified 191 patients from 33 countries with 72 unique mutations. Inclusion criteria included symptoms of immune dysregulation and a biochemically confirmed germline heterozygous GOF variant in STAT3.
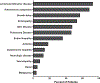
Results: Overall survival was 88%, median age at onset of symptoms was 2.3 years, and median age at diagnosis was 12 years. Immune dysregulatory features were present in all patients: lymphoproliferation was the most common manifestation (73%); increased frequencies of double-negative (CD4-CD8-) T cells were found in 83% of patients tested. Autoimmune cytopenias were the second most common clinical manifestation (67%), followed by growth delay, enteropathy, skin disease, pulmonary disease, endocrinopathy, arthritis, autoimmune hepatitis, neurologic disease, vasculopathy, renal disease, and malignancy. Infections were reported in 72% of the cohort. A cellular and humoral immunodeficiency was observed in 37% and 51% of patients, respectively. Clinical symptoms dramatically improved in patients treated with JAK inhibitors, while a variety of other immunomodulatory treatment modalities were less efficacious. Thus far, 23 patients have undergone bone marrow transplantation, with a 62% survival rate.
Conclusion: STAT3 GOF patients present with a wide array of immune-mediated disease including lymphoproliferation, autoimmune cytopenias, and multisystem autoimmunity. Patient care tends to be siloed, without a clear treatment strategy. Thus, early identification and prompt treatment implementation are lifesaving for STAT3 GOF syndrome.
Keywords: STAT3; autoimmunity; cytopenia; gain of function; immune dysregulation; immunodeficiency; lymphoproliferation; precision medicine.
Copyright © 2022 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: A. Kumar reports speakers’ bureau for SOBI; L. Forbes Satter reports consultancy for Enzyvant, Grifols, CSL Behring, Takeda, and ADMA; J. W. Leiding is a full-time employee and shareholder of Bluebirdbio and speaker and consultant for Sobi and Horizon Therapeutics; and T. Vogel has consulted for SOBI, Novartis, Pfizer, and Moderna. The rest of the authors declare that they have no relevant conflicts of interest.
Figures



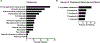
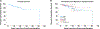
Comment in
-
A multifaceted disease: The stats of STAT3 GOF.J Allergy Clin Immunol. 2023 Apr;151(4):901-903. doi: 10.1016/j.jaci.2023.02.010. Epub 2023 Feb 21. J Allergy Clin Immunol. 2023. PMID: 36813184 No abstract available.
References
-
- Forbes LR, Vogel TP, Cooper MA, Castro-Wagner J, Schussler E, Weinacht KG, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol 2018;142:1665–9. - PMC - PubMed
-
- Hadjadj J, Aladjidi N, Fernandes H, Leverger G, Magérus-Chatinet A, Mazerolles F, et al. Pediatric Evans syndrome is associated with a high frequency of potentially damaging variants in immune genes. Blood 2019;134:9–21. - PubMed
-
- Heimall JR, Hagin D, Hajjar J, Henrickson SE, Hernandez-Trujillo HS, Tan Y, et al. Use of genetic testing for primary immunodeficiency patients. J Clin Immunol 2018;38:320–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

