Development of Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA)
- PMID: 36229046
- PMCID: PMC10069873
- DOI: 10.1183/13993003.00606-2022
Development of Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA)
Abstract
Background: Effectiveness studies with biological therapies for asthma lack standardised outcome measures. The COMSA (Core Outcome Measures sets for paediatric and adult Severe Asthma) Working Group sought to develop Core Outcome Measures (COM) sets to facilitate better synthesis of data and appraisal of biologics in paediatric and adult asthma clinical studies.
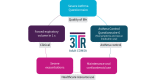
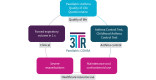
Methods: COMSA utilised a multi-stakeholder consensus process among patients with severe asthma, adult and paediatric clinicians, pharmaceutical representatives, and health regulators from across Europe. Evidence included a systematic review of development, validity and reliability of selected outcome measures plus a narrative review and a pan-European survey to better understand patients' and carers' views about outcome measures. It was discussed using a modified GRADE (Grading of Recommendations Assessment, Development and Evaluation) Evidence to Decision framework. Anonymous voting was conducted using predefined consensus criteria.
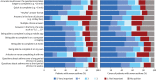
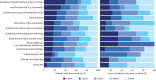
Results: Both adult and paediatric COM sets include forced expiratory volume in 1 s (FEV1) as z-scores, annual frequency of severe exacerbations and maintenance oral corticosteroid use. Additionally, the paediatric COM set includes the Paediatric Asthma Quality of Life Questionnaire and Asthma Control Test or Childhood Asthma Control Test, while the adult COM set includes the Severe Asthma Questionnaire and Asthma Control Questionnaire-6 (symptoms and rescue medication use reported separately).
Conclusions: This patient-centred collaboration has produced two COM sets for paediatric and adult severe asthma. It is expected that they will inform the methodology of future clinical trials, enhance comparability of efficacy and effectiveness of biological therapies, and help assess their socioeconomic value. COMSA will inform definitions of non-response and response to biological therapy for severe asthma.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: E. Khaleva and A. Rattu declare funding from 3TR European Union Innovative Medicines Initiative 2 to their institution for the present manuscript. C. Brightling declares grants from GlaxoSmithKline, AstraZeneca, Novartis, Chiesi, Boehringer Ingelheim, Genentech, Roche, Sanofi, Mologic and 4DPharma; consulting fees from GlaxoSmithKline, AstraZeneca, Novartis, Chiesi, Boehringer Ingelheim, Genentech, Roche, Sanofi, Mologic, 4DPharma and Teva; and support from the 3TR project. G.W. Clarke declares that he is an employee of AstraZeneca; and that he holds stock or stock options in AstraZeneca. M. van den Berge declares grants from GlaxoSmithKline, AstraZeneca, Roche, Genentech and Novartis paid to the university. A. Bossios declares honoraria for lectures from GlaxoSmithKline, AstraZeneca, Teva and Novartis; support for attending meetings from AstraZeneca and Novartis; honoraria for advisory board meetings from GlaxoSmithKline, AstraZeneca, Teva, Novartis and Sanofi; being a member of the Steering Committee of SHARP, Secretary of Assembly 5 (Airway Diseases, Asthma, COPD and Chronic Cough) of the European Respiratory Society; Vice-chair of the Nordic Severe Asthma Network (NSAN). V. Ramiconi and S. Romagosa Vilarnau declare unrestricted educational grants paid to the organisation from Novartis, Pfizer, AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, AbbVie, LeoPharma, Boehringer Ingelheim, Sanofi, Regeneron, OM Pharma, MSD, Roche and DBV Technologies; support for attending meetings from Novartis. S-E. Dahlén declares a 3TR Innovative Medicines Initiative grant; consulting fees for AstraZeneca, Cayman Co., GlaxoSmithKline, Novartis, Regeneron, Sanofi and Teva; payment for lectures from AstraZeneca and Sanofi. S. Principe declares support for provision of study materials and medical writing. G. Hedlin declares participation in advisory boards of AstraZeneca and Sanofi. F. Singer reports grants from Lung League Bern, grants from the Swiss Cystic Fibrosis Society (CFCH), personal fees from Vertex Pharmaceuticals, personal fees from Novartis, outside the submitted work. A. Deschildre reports personal consulting fees from Sanofi-Regeneron, ALK, Novartis and GlaxoSmithKline; honoraria for lectures from ALK, Boehringer Ingelheim and Novartis; support for attending meetings from AstraZeneca, Stallergènes-Greer, MEDA, Nutricia, Sanofi and Novartis, outside the submitted work; and being on the scientific committee of SFA (Société Française d'Allergologie). L.J. Fleming declares participation in advisory boards and honoraria for lectures from Sanofi, Respiri UK, AstraZeneca, Novartis and Teva, outside of the scope of this publication; all payments were made to her institution. H. Staudinger reports that he is a salaried employee of Sanofi Genzyme and owns company stock of Sanofi and Merck & Co. K.C. Pike declares consultancy fees from Novartis, Adherium and Respiri, and honoraria for a lecture from Novartis. J. Grigg declares payments from GlaxoSmithKline, OM Pharma, Omron and Novartis (advisory boards), Sanofi (for lectures) and AstraZeneca (CI clinical trial). N. Rutjes reports personal fees for advisory board work from Sanofi. G.H. Koppelman reports receiving research grants from the Lung Foundation of the Netherlands, Ubbo Emmius Foundation, H2020 European Union, Teva, GlaxoSmithKline and Vertex, outside this work (money to institution); he reports memberships of advisory boards to GlaxoSmithKline and PURE-IMS, outside this work (money to institution). D. Cunoosamy holds shares in AstraZeneca and Sanofi. A.H. Maitland-van der Zee has received research grants outside the submitted work from GlaxoSmithKline, Boehringer Ingelheim and Vertex, she is the PI of a P4O2 (Precision Medicine for more Oxygen) public private partnership sponsored by Health Holland involving many private partners that contribute in cash and/or in kind (Boehringer Ingelheim, Breathomix, Fluidda, Ortec Logiqcare, Philips, Quantib-U, Roche, Smartfish, SODAQ, Thirona, TopMD and Novartis), and she has served in advisory boards for AstraZeneca, GlaxoSmithKline and Boehringer Ingelheim with money paid to her institution. K.F. Chung has received honoraria for participating in advisory board meetings of GlaxoSmithKline, AstraZeneca, Roche, Novartis, Merck and Shionogi, regarding treatments for asthma, COPD and chronic cough, and has also been remunerated for speaking engagements for Novartis and AstraZeneca; received a MRC grant on Precision Medicine for severe asthma, EPSRC grant on air pollution and asthma, and a GlaxoSmithKline grant on mepolizumab and eosinophils in asthma. P. Nagakumar received speaker fees for talks on severe asthma from GlaxoSmithKline and Novartis. G. Brusselle declares payments from AstraZeneca, Novartis, Boehringer Ingelheim, Chiesi, Sanofi, GlaxoSmithKline and MSD, outside the submitted work. E. Hamelmann declares support from the German Ministry of Education and Research (BMBF) and German Asthma Net (GAN) eV; funding for research in severe asthma in children (CHAMP-01GL1742D) and for Severe Asthma Register. S. Vijverberg is PI of the PERMEABLE consortium. The PERMEABLE consortium is a research consortium focused on severe asthma and allergy and supported by ZonMW (456008004), the Swedish Research Council (2018-05619), the Ministry of Education, Science and Sport of the Republic of Slovenia (C3330-19-252012), and the German Ministry of Education and Research (BMBF) FKZ01KU1909A), under the frame of the ERA PerMed JTC 2018 Call. In addition, S. Vijverberg has received research funding for a project on severe paediatric asthma from the Lung Foundation Netherlands (6.2.18.244JO). A-M.M. Schoos has participated on an advisory board for ALK. B. Dahlén reports personal fees for lectures from AstraZeneca, Novartis and Sanofi; and grants from Novartis and GlaxoSmithKline, outside the submitted work; participation on an advisory board for AstraZeneca and Sanofi. A. Exley declares being a minority shareholder in GlaxoSmithKline Plc. E.A. Gaillard reports consultancy work for Boehringer Ingelheim with money paid to the institution (University of Leicester); investigator-led research grants from Circassia Group, Gilead Sciences, Chiesi Limited and Propeller Health; and has a research collaboration with Medimmune. M. Pijnenburg declares payments to her institution from Sanofi Genzyme (advisory work) and Novartis (speakers fee). E. Melén declares consulting fees from AstraZeneca, Chiesi, Novartis and Sanofi, outside the submitted work. R. Chaudhuri has received lecture fees from GlaxoSmithKline, AstraZeneca, Teva, Chiesi, Sanofi and Novartis; honoraria for advisory board meetings from GlaxoSmithKline, AstraZeneca, Teva, Chiesi and Novartis; sponsorship to attend international scientific meetings from Chiesi, Napp, Sanofi and GlaxoSmithKline, and a research grant to her institute from AstraZeneca for a UK multi-centre study. C. Pilette declares grants, consulting fees and honoraria for lectures (paid to institution) from AstraZeneca, Chiesi, GlaxoSmithKline, Novartis, Mundipharma, Teva, Sanofi and ALK. C. Porsbjerg declares grants (paid to institution), consulting fees (paid to institution and personal honoraria) and honoraria for lectures (paid to institution and personal honoraria) from AstraZeneca, GlaxoSmithKline, Novartis, Teva, Sanofi, Chiesi and ALK; participation on an advisory board (paid to institution and personal honoraria) for AstraZeneca, Novartis, Teva, Sanofi and ALK. C. Coleman and C. Williams declare funding received to support this work by the European Lung Foundation (ELF) from the European Commission's Innovative Medicines Initiative 2 Joint Undertaking under grant agreement number 831434 (3TR), and are employees of the ELF. B. Ahrens, S. Kaul, D. Hartenstein and V. Mahler declare no conflict of interest for this article and state that the views expressed in this review are the personal views of the author and may not be understood or quoted as being made on behalf of or reflecting the position of the respective national competent authorities, the European Medicines Agency or one of its committees or working parties. L.G. Heaney declares support from the 3TR; grants from industrial pharma partners Amgen, AstraZeneca, Medimmune, Janssen, Novartis, Roche/Genentech, GlaxoSmithKline and Boehringer Ingelheim; project grant funding from Medimmune, Novartis UK, Roche/Genentech and GlaxoSmithKline, outside the submitted work; payments for lectures by AstraZeneca, Novartis, Roche/Genentech, GlaxoSmithKline, Chiesi and Teva, outside the submitted work; travel funding support to international respiratory meetings by AstraZeneca, Chiesi, Novartis, Boehringer Ingelheim, Teva and GlaxoSmithKline, outside the submitted work; advisory boards for AstraZeneca, Novartis, GlaxoSmithKline, Chiesi, Teva, Theravance and Vectura, outside the submitted work. R. Djukanovic declares funding from the European Respiratory Society, Teva, GlaxoSmithKline, Novartis, Sanofi and Chiesi for the SHARP Clinical Research Collaboration; consulting fees for Synairgen; honorarium for a lecture from GlaxoSmithKline; participation on a data safety monitoring board or advisory board for Kymab (Cambridge) and shares in Synairgen outside of the submitted work. A. Bourdin declares grants from Boehringer and AstraZeneca; consulting fees and payments from Boehringer, AstraZeneca, GlaxoSmithKline, Novartis, Chiesi, Regeneron, Sanofi and Amgen outside of the submitted work. B. Karadag declares participation in a trial conducted by Sanofi (payment to institution) and attending advisory board meetings for GlaxoSmithKline (personal fees). K. Blumchen declares grants from Aimmune Therapeutics, DBV Technologies and Hipp GmbH; consulting fees from Aimmune Therapeutics, DBV Technologies and Allergy Therapeutics; payments for lectures from Aimmune Therapeutics, DBV Technologies, Novartis, Allergy Therapeutics, HAL, ALK, Allergopharma, Nutricia, Thermo Fisher Scientific, and Bausch and Lomb; personal fees for expert discussions from Novartis and Nestle; fees for attending meetings from Aimmune Therapeutics and DBV Technologies; being on data safety monitoring board of Charité, IIT. A. Gupta received speaker/advisory board fees from GlaxoSmithKline, Novartis, AstraZeneca and Boehringer Ingelheim; received research grants from GlaxoSmithKline, Novartis, AstraZeneca and Boehringer Ingelheim, paid to institution. L. Giovannini-Chami declares consulting fees from ALK, AstraZeneca and Novartis; honoraria for lectures, presentations from Novartis, ALK, Stallergènes, Sanofi and AstraZeneca; support for attending meetings from Stallergènes; participation on a data safety monitoring board or advisory board for Sanofi; being head of the Scientific Committee of the French Pediatric Pulmonology and Allergology Society. C.S. Murray has received lecture fees from GlaxoSmithKline and Novartis; received grants from Asthma UK and National Institute for Health Research; and has participated on an advisory board for Boehringer Ingelheim. G. Roberts declares European Union Innovative Medicines Initiative funding and AstraZeneca paid to the institution. Other co-authors declare no conflicts of interest for this article.
Figures

Comment in
-
Moving towards patient-centred outcomes: the Severe Asthma Questionnaire.Eur Respir J. 2023 May 5;61(5):2202305. doi: 10.1183/13993003.02305-2022. Print 2023 May. Eur Respir J. 2023. PMID: 36585255 No abstract available.
-
Defining the questions to be asked in severe asthma trials: data from the COMSA working group.Eur Respir J. 2023 Apr 3;61(4):2202058. doi: 10.1183/13993003.02058-2022. Print 2023 Apr. Eur Respir J. 2023. PMID: 37012083 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
