In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes
- PMID: 36229047
- PMCID: PMC9909213
- DOI: 10.1183/13993003.00910-2022
In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes
Abstract
Background: Critically ill patients routinely receive antibiotics with activity against anaerobic gut bacteria. However, in other disease states and animal models, gut anaerobes are protective against pneumonia, organ failure and mortality. We therefore designed a translational series of analyses and experiments to determine the effects of anti-anaerobic antibiotics on the risk of adverse clinical outcomes among critically ill patients.
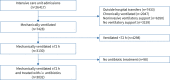
Methods: We conducted a retrospective single-centre cohort study of 3032 critically ill patients, comparing patients who did and did not receive early anti-anaerobic antibiotics. We compared intensive care unit outcomes (ventilator-associated pneumonia (VAP)-free survival, infection-free survival and overall survival) in all patients and changes in gut microbiota in a subcohort of 116 patients. In murine models, we studied the effects of anaerobe depletion in infectious (Klebsiella pneumoniae and Staphylococcus aureus pneumonia) and noninfectious (hyperoxia) injury models.
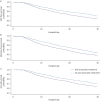
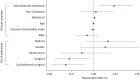
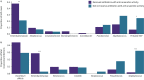
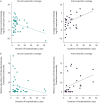
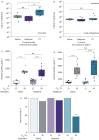
Results: Early administration of anti-anaerobic antibiotics was associated with decreased VAP-free survival (hazard ratio (HR) 1.24, 95% CI 1.06-1.45), infection-free survival (HR 1.22, 95% CI 1.09-1.38) and overall survival (HR 1.14, 95% CI 1.02-1.28). Patients who received anti-anaerobic antibiotics had decreased initial gut bacterial density (p=0.00038), increased microbiome expansion during hospitalisation (p=0.011) and domination by Enterobacteriaceae spp. (p=0.045). Enterobacteriaceae were also enriched among respiratory pathogens in anti-anaerobic-treated patients (p<2.2×10-16). In murine models, treatment with anti-anaerobic antibiotics increased susceptibility to Enterobacteriaceae pneumonia (p<0.05) and increased the lethality of hyperoxia (p=0.0002).
Conclusions: In critically ill patients, early treatment with anti-anaerobic antibiotics is associated with increased mortality. Mechanisms may include enrichment of the gut with respiratory pathogens, but increased mortality is incompletely explained by infections alone. Given consistent clinical and experimental evidence of harm, the widespread use of anti-anaerobic antibiotics should be reconsidered.
The content of this work is not subject to copyright. Design and branding are copyright ©ERS 2023.
Conflict of interest statement
Conflict of interest: All authors have nothing to disclose.
Figures

Comment in
-
Empiric anti-anaerobic antibiotics are associated with adverse clinical outcomes in emergency department patients.Eur Respir J. 2023 May 11;61(5):2300413. doi: 10.1183/13993003.00413-2023. Print 2023 May. Eur Respir J. 2023. PMID: 37169379 No abstract available.
-
Anti-anaerobic antibiotics: indication is key.Eur Respir J. 2023 May 11;61(5):2300318. doi: 10.1183/13993003.00318-2023. Print 2023 May. Eur Respir J. 2023. PMID: 37169380 No abstract available.
-
Reply to: Anti-anaerobic antibiotics: indication is key.Eur Respir J. 2023 May 11;61(5):2300492. doi: 10.1183/13993003.00492-2023. Print 2023 May. Eur Respir J. 2023. PMID: 37169381 No abstract available.
Similar articles
-
Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?J Trauma. 2009 May;66(5):1343-8. doi: 10.1097/TA.0b013e31819dca4e. J Trauma. 2009. PMID: 19430237
-
Empiric antibiotics pending bronchoalveolar lavage data in patients without pneumonia significantly alters the flora, but not the resistance profile, if a subsequent pneumonia develops.J Surg Res. 2013 May;181(2):323-8. doi: 10.1016/j.jss.2012.07.021. Epub 2012 Jul 26. J Surg Res. 2013. PMID: 22906560
-
Trauma and Antimicrobial Resistance Are Independent Predictors of Inadequate Empirical Antimicrobial Treatment of Ventilator-Associated Pneumonia in Critically Ill Patients.Surg Infect (Larchmt). 2021 Sep;22(7):730-737. doi: 10.1089/sur.2020.306. Epub 2021 Jan 13. Surg Infect (Larchmt). 2021. PMID: 33439780
-
Chlorhexidine bathing of the critically ill for the prevention of hospital-acquired infection.Cochrane Database Syst Rev. 2019 Aug 30;8(8):CD012248. doi: 10.1002/14651858.CD012248.pub2. Cochrane Database Syst Rev. 2019. PMID: 31476022 Free PMC article.
-
Novel investigational treatments for ventilator-associated pneumonia and critically ill patients in the intensive care unit.Expert Opin Investig Drugs. 2022 Feb;31(2):173-192. doi: 10.1080/13543784.2022.2030312. Epub 2022 Feb 2. Expert Opin Investig Drugs. 2022. PMID: 35040388 Review.
Cited by
-
Inconsistencies in the Indian Guidelines for the Prescription of Antibiotics for Critically Ill Patients.Indian J Crit Care Med. 2024 Oct;28(10):908-911. doi: 10.5005/jp-journals-10071-24812. Epub 2024 Sep 30. Indian J Crit Care Med. 2024. PMID: 39411298 Free PMC article.
-
Effects of Tanreqing injection on the gut microbiota in healthy volunteers.Front Cell Infect Microbiol. 2024 Oct 4;14:1428476. doi: 10.3389/fcimb.2024.1428476. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39431053 Free PMC article.
-
How to Understand a Revolution: Guts, Lungs, and Bronchiectasis.Am J Respir Crit Care Med. 2023 Apr 1;207(7):812-814. doi: 10.1164/rccm.202211-2179ED. Am J Respir Crit Care Med. 2023. PMID: 36473273 Free PMC article. No abstract available.
-
The Gut Microbiome Modulates Body Temperature Both in Sepsis and Health.Am J Respir Crit Care Med. 2023 Apr 15;207(8):1030-1041. doi: 10.1164/rccm.202201-0161OC. Am J Respir Crit Care Med. 2023. PMID: 36378114 Free PMC article.
-
Robust airway microbiome signatures in acute respiratory failure and hospital-acquired pneumonia.Nat Med. 2023 Nov;29(11):2793-2804. doi: 10.1038/s41591-023-02617-9. Epub 2023 Nov 13. Nat Med. 2023. PMID: 37957375
References
-
- Ewig S, Torres A, El-Ebiary M, et al. . Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med 1999; 159: 188–198. doi:10.1164/ajrccm.159.1.9803097 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
