Clinical outcomes of bronchiectasis in India: data from the EMBARC/Respiratory Research Network of India registry
- PMID: 36229049
- PMCID: PMC9816417
- DOI: 10.1183/13993003.00611-2022
Clinical outcomes of bronchiectasis in India: data from the EMBARC/Respiratory Research Network of India registry
Abstract
Background: Identifying risk factors for poor outcomes can help with risk stratification and targeting of treatment. Risk factors for mortality and exacerbations have been identified in bronchiectasis but have been almost exclusively studied in European and North American populations. This study investigated the risk factors for poor outcome in a large population of bronchiectasis patients enrolled in India.
Methods: The European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India (EMBARC-India) registry is a prospective observational study of adults with computed tomography-confirmed bronchiectasis enrolled at 31 sites across India. Baseline characteristics of patients were used to investigate associations with key clinical outcomes: mortality, severe exacerbations requiring hospital admission, overall exacerbation frequency and decline in forced expiratory volume in 1 s.
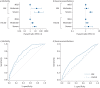
Results: 1018 patients with at least 12-month follow-up data were enrolled in the follow-up study. Frequent exacerbations (≥3 per year) at baseline were associated with an increased risk of mortality (hazard ratio (HR) 3.23, 95% CI 1.39-7.50), severe exacerbations (HR 2.71, 95% CI 1.92-3.83), future exacerbations (incidence rate ratio (IRR) 3.08, 95% CI 2.36-4.01) and lung function decline. Coexisting COPD, dyspnoea and current cigarette smoking were similarly associated with a worse outcome across all end-points studied. Additional predictors of mortality and severe exacerbations were increasing age and cardiovascular comorbidity. Infection with Gram-negative pathogens (predominantly Klebsiella pneumoniae) was independently associated with increased mortality (HR 3.13, 95% CI 1.62-6.06), while Pseudomonas aeruginosa infection was associated with severe exacerbations (HR 1.41, 95% CI 1.01-1.97) and overall exacerbation rate (IRR 1.47, 95% CI 1.13-1.91).
Conclusions: This study identifies risk factors for morbidity and mortality among bronchiectasis patients in India. Identification of these risk factors may support treatment approaches optimised to an Asian setting.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: M. Loebinger reports grants from AstraZeneca, Insmed and Grifols, outside the submitted work. F. Blasi reports grants from AstraZeneca, Bayer, Chiesi, GlaxoSmithKline, Menarini and Pfizer; consulting fees from Novartis, Pfizer, Zambon, Vertex, Viatris, AstraZeneca, Bayer, Chiesi, GlaxoSmithKline, Grifols, Guidotti, Insmed and Menarini, outside the submitted work. A. Shoemark reports grants from AstraZeneca, outside the submitted work. E. Polverino reports grants from Chiesi, Zambon, Shionogi, Teva, CSL Boehring, Insmed and Grifols, outside the submitted work. T. Welte reports grants from AstraZeneca and GlaxoSmithKline, outside the submitted work. M. Shteinberg reports grants from Trudell, GlaxoSmithKline, Novartis, Boehringer Ingelheim, AstraZeneca, Kamada, Vertex, Teva, Actelion and Rafa, outside the submitted work. S. Aliberti reports grants from Insmed, Chiesi and Fisher & Paykel; consulting fees from McGraw Hill, Insmed, Zambon, AstraZeneca, CSL Behring, Grifols, Fondazione Charta, Boehringer Ingelheim, Chiesi, Zcube, Menarini and GlaxoSmithKline, outside the submitted work. S. Limaye reports grants from Glenmark, supporting the present manuscript. J.D. Chalmers reports grants from AstraZeneca, Novartis, Boehringer Ingelheim, Insmed, GlaxoSmithKline and Gilead Sciences; consulting fees from AstraZeneca, Insmed, Boehringer Ingelheim, Janssen, Chiesi, Novartis, GlaxoSmithKline, Pfizer and Zambon, outside the submitted work. All other authors have nothing to disclose.
Figures
Comment in
-
Bronchiectasis in low- and middle-income countries: the importance of the wider view.Eur Respir J. 2023 Jan 6;61(1):2201977. doi: 10.1183/13993003.01977-2022. Print 2023 Jan. Eur Respir J. 2023. PMID: 36609519 No abstract available.
-
Reply to: Insights into the clinical outcomes of bronchiectasis.Eur Respir J. 2023 Feb 2;61(2):2202224. doi: 10.1183/13993003.02224-2022. Print 2023 Feb. Eur Respir J. 2023. PMID: 36731902 No abstract available.
-
Insights into the clinical outcomes of bronchiectasis.Eur Respir J. 2023 Feb 2;61(2):2202104. doi: 10.1183/13993003.02104-2022. Print 2023 Feb. Eur Respir J. 2023. PMID: 36731904 No abstract available.
References
-
- Zhou Y, Wang C, Yao W, et al. . [The prevalence and risk factors of bronchiectasis in residents aged 40 years old and above in seven cities in China]. Zhonghua nei ke za zhi 2013; 52: 379–382. - PubMed
