Intensive Blood Pressure Lowering in Patients With Malignant Left Ventricular Hypertrophy
- PMID: 36229087
- PMCID: PMC9982833
- DOI: 10.1016/j.jacc.2022.08.735
Intensive Blood Pressure Lowering in Patients With Malignant Left Ventricular Hypertrophy
Abstract
Background: Left ventricular hypertrophy (LVH) combined with elevations in cardiac biomarkers reflecting myocardial injury and neurohormonal stress (malignant LVH) is associated with a high risk for heart failure and death.
Objectives: The aim of this study was to determine the impact of intensive systolic blood pressure (SBP) control on the prevention of malignant LVH and its consequences.
Methods: A total of 8,820 participants in SPRINT (Systolic Blood Pressure Intervention Trial) were classified into groups based on the presence or absence of LVH assessed by 12-lead ECG, and elevations in biomarker levels (high-sensitivity cardiac troponin T ≥14 ng/L or N-terminal pro-B-type natriuretic peptide ≥125 pg/mL) at baseline. The effects of intensive vs standard SBP lowering on rates of acute decompensated heart failure (ADHF) events and death and on the incidence and regression of malignant LVH were determined.
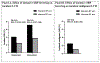
Results: Randomization to intensive SBP lowering led to similar relative reductions in ADHF events and death across the combined LVH/biomarker groups (P for interaction = 0.68). The absolute risk reduction over 4 years in ADHF events and death was 4.4% (95% CI: -5.2% to 13.9%) among participants with baseline malignant LVH (n = 449) and 1.2% (95% CI: 0.0%-2.5%) for those without LVH and nonelevated biomarkers (n = 4,361). Intensive SBP lowering also reduced the incidence of malignant LVH over 2 years (2.5% vs 1.1%; OR: 0.44; 95% CI: 0.30-0.63).
Conclusions: Intensive SBP lowering prevented malignant LVH and may provide substantial absolute risk reduction in the composite of ADHF events and death among SPRINT participants with baseline malignant LVH.
Keywords: heart failure; hypertension; malignant LVH; natriuretic peptide; troponin.
Copyright © 2022 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This ancillary study was supported by the National Heart, Lung, and Blood Institute (1R01HL144112-01 for Dr Berry). Analytical reagents for hs-cTnT and NT-proBNP measurements were donated by Roche. SPRINT was sponsored by the National Institutes of Health, including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13–002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, or the United States Government. Support was also received from the following CTSAs funded by the National Center for Advancing Translational Sciences: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134 and UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 and UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017–06, University of Utah: UL1TR000105–05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of California, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420. Dr de Lemos has received grant support from Roche Diagnostics and Abbott Diagnostics; has received consulting fees from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Quidel Cardiovascular, Inc, and Siemen’s Health Care Diagnostics; and has been named a co-owner on a patent awarded to the University of Maryland (US Patent Application Number: 15/309,754) entitled: “Methods for Assessing Differential Risk for Developing Heart Failure.” Dr Kitzman has received honoraria as a consultant outside the present study from Bayer, Merck, Pfizer, Corvia Medical, Boehringer Ingelheim, Ketyo, Rivus, Novo Nordisk, AstraZeneca, and Novartis; has received grant funding from Novartis, Bayer, Pfizer, Novo Nordisk, and AstraZeneca; and has stock ownership in Gilead Sciences. Dr Berry has received grant support from the National Institutes of Health, Roche Diagnostics, and Abbott Diagnostics; and has received consulting fees from Roche Diagnostics and the Cooper Institute. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


Comment in
-
Malignant Hypertensive Cardiomyopathy: Definition Matters.J Am Coll Cardiol. 2023 Feb 28;81(8):e57-e58. doi: 10.1016/j.jacc.2022.11.058. J Am Coll Cardiol. 2023. PMID: 36813381 No abstract available.
-
Reply: Malignant Hypertensive Cardiomyopathy: Definition Matters.J Am Coll Cardiol. 2023 Feb 28;81(8):e59. doi: 10.1016/j.jacc.2022.12.015. J Am Coll Cardiol. 2023. PMID: 36813382 No abstract available.
References
-
- Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. - DOI - PubMed
-
- Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. - DOI - PubMed
-
- Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- R01 HL144112/HL/NHLBI NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

