Temporal Dynamics of MOG Antibodies in Children With Acquired Demyelinating Syndrome
- PMID: 36229191
- PMCID: PMC9562044
- DOI: 10.1212/NXI.0000000000200035
Temporal Dynamics of MOG Antibodies in Children With Acquired Demyelinating Syndrome
Abstract
Background and objective: The spectrum of myelin oligodendrocyte glycoprotein (MOG) antibody-associated disorder (MOGAD) comprises monophasic diseases such as acute disseminated encephalomyelitis (ADEM), optic neuritis (ON), and transverse myelitis and relapsing courses of these presentations. Persistently high MOG antibodies (MOG immunoglobulin G [IgG]) are found in patients with a relapsing disease course. Prognostic factors to determine the clinical course of children with a first MOGAD are still lacking. The objective of the study is to assess the clinical and laboratory prognostic parameters for a risk of relapse and the temporal dynamics of MOG-IgG titers in children with MOGAD in correlation with clinical presentation and disease course.
Methods: In this prospective multicenter hospital-based study, children with a first demyelinating attack and complete data set comprising clinical and radiologic findings, MOG-IgG titer at onset, and clinical and serologic follow-up data were included. Serum samples were analyzed by live cell-based assay, and a titer level of ≥1:160 was classified as MOG-IgG-positive.
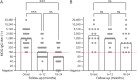
Results: One hundred sixteen children (f:m = 57:59) with MOGAD were included and initially diagnosed with ADEM (n = 59), unilateral ON (n = 12), bilateral ON (n = 16), myelitis (n = 6), neuromyelitis optica spectrum disorder (n = 8) or encephalitis (n = 6). The median follow-up time was 3 years in monophasic and 5 years in relapsing patients. There was no significant association between disease course and MOG-IgG titers at onset, sex, age at presentation, or clinical phenotype. Seroconversion to MOG-IgG-negative within 2 years of the initial event showed a significant risk reduction for a relapsing disease course. Forty-two/one hundred sixteen patients (monophasic n = 26, relapsing n = 16) had serial MOG-IgG testing in years 1 and 2 after the initial event. In contrast to relapsing patients, monophasic patients showed a significant decrease of MOG-IgG titers during the first and second years, often with seroconversion to negative titers. During the follow-up, MOG-IgG titers were persistently higher in relapsing than in monophasic patients. Decrease in MOG-IgG of ≥3 dilution steps after the first and second years was shown to be associated with a decreased risk of relapses. In our cohort, no patient experienced a relapse after seroconversion to MOG-IgG-negative.
Discussion: In this study, patients with declining MOG-IgG titers, particularly those with seroconversion to MOG-IgG-negative, are shown to have a significantly reduced relapse risk.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Hennes EM, Baumann M, Schanda K, et al. . Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89(9):900-908. - PubMed
-
- Baumann M, Hennes EM, Schanda K, et al. . Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult Scler. 2016;22(14):1821-1829. - PubMed
-
- Lechner C, Baumann M, Hennes EM, et al. . Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J Neurol Neurosurg Psychiatry. 2016;87(8):897-905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
