[2]-Ladderanes as isosteres for meta-substituted aromatic rings and rigidified cyclohexanes
- PMID: 36229621
- PMCID: PMC9561113
- DOI: 10.1038/s41467-022-33827-3
[2]-Ladderanes as isosteres for meta-substituted aromatic rings and rigidified cyclohexanes
Abstract
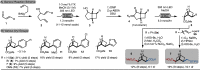
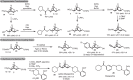
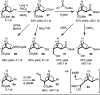
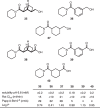
Aromatic ring isosteres and rigidified saturated hydrocarbons are important motifs to enable drug discovery. Herein we disclose [2]-ladderanes as a class of meta-substituted aromatic ring isosteres and rigidified cyclohexanes. A straightforward synthesis of the building blocks is presented along with representative derivatization. Preliminary studies reveal that the [2]-ladderanes offer similar metabolic and physicochemical properties thus establishing this class of molecules as interesting motifs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
(Bio)isosteres of ortho- and meta-substituted benzenes.Beilstein J Org Chem. 2024 Apr 19;20:859-890. doi: 10.3762/bjoc.20.78. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38655554 Free PMC article. Review.
-
3-Oxabicyclo[3.1.1]heptanes as Isosteres of meta-Substituted Benzene Rings.Org Lett. 2025 Apr 4;27(13):3291-3295. doi: 10.1021/acs.orglett.5c00646. Epub 2025 Mar 20. Org Lett. 2025. PMID: 40113336
-
A Photochemical Strategy for the Conversion of Nitroarenes into Rigidified Pyrrolidine Analogues.J Am Chem Soc. 2023 Dec 20;145(50):27810-27820. doi: 10.1021/jacs.3c10863. Epub 2023 Dec 7. J Am Chem Soc. 2023. PMID: 38059920
-
Silver(I)-Catalyzed Synthesis of Cuneanes from Cubanes and their Investigation as Isosteres.J Am Chem Soc. 2023 Aug 2;145(30):16365-16373. doi: 10.1021/jacs.3c03207. Epub 2023 Jul 21. J Am Chem Soc. 2023. PMID: 37478562 Free PMC article.
-
The Emergence and Properties of Selectively Fluorinated 'Janus' Cyclohexanes.Chem Rec. 2023 Sep;23(9):e202300027. doi: 10.1002/tcr.202300027. Epub 2023 Apr 4. Chem Rec. 2023. PMID: 37016509 Review.
Cited by
-
(Bio)isosteres of ortho- and meta-substituted benzenes.Beilstein J Org Chem. 2024 Apr 19;20:859-890. doi: 10.3762/bjoc.20.78. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38655554 Free PMC article. Review.
-
2,5-disubstituted bicyclo[2.1.1]hexanes as rigidified cyclopentane variants.Chem Sci. 2023 Jul 12;14(30):8070-8075. doi: 10.1039/d3sc02695g. eCollection 2023 Aug 2. Chem Sci. 2023. PMID: 37538817 Free PMC article.
-
Exploring Cuneanes as Potential Benzene Isosteres and Energetic Materials: Scope and Mechanistic Investigations into Regioselective Rearrangements from Cubanes.J Am Chem Soc. 2023 Aug 2;145(30):16355-16364. doi: 10.1021/jacs.3c03226. Epub 2023 Jul 24. J Am Chem Soc. 2023. PMID: 37486221 Free PMC article.
-
General access to cubanes as benzene bioisosteres.Nature. 2023 Jun;618(7965):513-518. doi: 10.1038/s41586-023-06021-8. Epub 2023 Apr 4. Nature. 2023. PMID: 37015289 Free PMC article.
-
Three-dimensional saturated C(sp3)-rich bioisosteres for benzene.Nat Rev Chem. 2024 Aug;8(8):605-627. doi: 10.1038/s41570-024-00623-0. Epub 2024 Jul 9. Nat Rev Chem. 2024. PMID: 38982260 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

