Retrosplenial and subicular inputs converge on superficially projecting layer V neurons of medial entorhinal cortex
- PMID: 36229654
- PMCID: PMC9618507
- DOI: 10.1007/s00429-022-02578-8
Retrosplenial and subicular inputs converge on superficially projecting layer V neurons of medial entorhinal cortex
Abstract
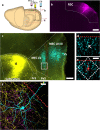
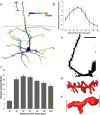
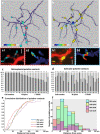
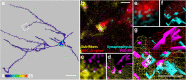
The medial entorhinal cortex (MEC) plays a pivotal role in spatial processing together with hippocampal formation. The retrosplenial cortex (RSC) is also implicated in this process, and it is thus relevant to understand how these structures interact. This requires precise knowledge of their connectivity. Projections from neurons in RSC synapse onto principal neurons in layer V of MEC and some of these neurons send axons into superficial layers of MEC. Layer V of MEC is also the main target for hippocampal efferents from the subiculum and CA1 field. The aim of this study was to assess whether the population of cells targeted by RSC projections also receives input from the hippocampal formation and to compare the distribution of synaptic contacts on target dendrites. We labeled the cells in layer V of MEC by injecting a retrograde tracer into superficial layers. At the same time, we labeled RSC and subicular projections with different anterograde tracers. 3D-reconstruction of the labeled cells and axons revealed likely synaptic contacts between presynaptic boutons of both origins and postsynaptic MEC layer V basal dendrites. Moreover, these contacts overlapped on the same dendritic segments without targeting specific domains. Our results support the notion that MEC layer V neurons that project to the superficial layers receive convergent input from both RSC and subiculum. These data thus suggest that convergent subicular and RSC information contributes to the signal that neurons in superficial layers of EC send to the hippocampal formation.
Keywords: Confocal microscopy; Entorhinal cortex; Hippocampal-entorhinal projections; Input convergence; Intrinsic circuitry; Neuroanatomical tracing.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Superficially projecting principal neurons in layer V of medial entorhinal cortex in the rat receive excitatory retrosplenial input.J Neurosci. 2013 Oct 2;33(40):15779-92. doi: 10.1523/JNEUROSCI.2646-13.2013. J Neurosci. 2013. PMID: 24089485 Free PMC article.
-
Input from the presubiculum to dendrites of layer-V neurons of the medial entorhinal cortex of the rat.Brain Res. 2004 Jul 2;1013(1):1-12. doi: 10.1016/j.brainres.2004.03.017. Brain Res. 2004. PMID: 15196963
-
Presubiculum layer III conveys retrosplenial input to the medial entorhinal cortex.Hippocampus. 2012 Apr;22(4):881-95. doi: 10.1002/hipo.20949. Epub 2011 Jun 24. Hippocampus. 2012. PMID: 21710546
-
Functional connectivity of the entorhinal-hippocampal space circuit.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 23;369(1635):20120516. doi: 10.1098/rstb.2012.0516. Print 2014 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24366130 Free PMC article. Review.
-
Species differences in the projections from the entorhinal cortex to the hippocampus.Brain Res Bull. 2002 Feb-Mar 1;57(3-4):553-6. doi: 10.1016/s0361-9230(01)00683-9. Brain Res Bull. 2002. PMID: 11923027 Review.
Cited by
-
Retrosplenial inputs drive diverse visual representations in the medial entorhinal cortex.bioRxiv [Preprint]. 2023 Oct 4:2023.10.03.560642. doi: 10.1101/2023.10.03.560642. bioRxiv. 2023. Update in: Cell Rep. 2024 Jul 23;43(7):114470. doi: 10.1016/j.celrep.2024.114470. PMID: 37873152 Free PMC article. Updated. Preprint.
-
Architecture of the Entorhinal Cortex A Review of Entorhinal Anatomy in Rodents with Some Comparative Notes.Front Syst Neurosci. 2017 Jun 28;11:46. doi: 10.3389/fnsys.2017.00046. eCollection 2017. Front Syst Neurosci. 2017. PMID: 28701931 Free PMC article. Review.
-
FOS mapping reveals two complementary circuits for spatial navigation in mouse.Sci Rep. 2024 Sep 11;14(1):21252. doi: 10.1038/s41598-024-72272-8. Sci Rep. 2024. PMID: 39261637 Free PMC article.
-
Retrosplenial inputs drive visual representations in the medial entorhinal cortex.Cell Rep. 2024 Jul 23;43(7):114470. doi: 10.1016/j.celrep.2024.114470. Epub 2024 Jul 9. Cell Rep. 2024. PMID: 38985682 Free PMC article.
-
Allocentric and egocentric spatial representations coexist in rodent medial entorhinal cortex.Nat Commun. 2025 Jan 3;16(1):356. doi: 10.1038/s41467-024-54699-9. Nat Commun. 2025. PMID: 39753542 Free PMC article.
References
-
- Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40(4):239–257. doi: 10.1002/syn.1047. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

