The race to develop oral SERDs and other novel estrogen receptor inhibitors: recent clinical trial results and impact on treatment options
- PMID: 36229710
- PMCID: PMC9560885
- DOI: 10.1007/s10555-022-10066-y
The race to develop oral SERDs and other novel estrogen receptor inhibitors: recent clinical trial results and impact on treatment options
Abstract
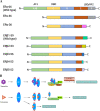
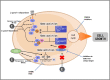
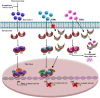
Hormonal therapy plays a vital part in the treatment of estrogen receptor-positive (ER +) breast cancer. ER can be activated in a ligand-dependent and independent manner. Currently available ER-targeting agents include selective estrogen receptor modulators (SERMs), selective estrogen receptor degraders (SERDs), and aromatase inhibitors (AIs). Estrogen receptor mutation (ESR1 mutation) is one of the common mechanisms by which breast cancer becomes resistant to additional therapies from SERMs or AIs. These tumors remain sensitive to SERDs such as fulvestrant. Fulvestrant is limited in clinical utilization by its intramuscular formulation and once-monthly injection in large volumes. Oral SERDs are being rapidly developed to replace fulvestrant with the potential of higher efficacy and lower toxicities. Elacestrant is the first oral SERD that went through a randomized phase III trial showing increased efficacy, especially in tumors bearing ESR1 mutation, and good tolerability. Two other oral SERDs recently failed to achieve the primary endpoints of longer progression-free survival (PFS). They targeted tumors previously treated with several lines of prior therapies untested for ESR1 mutation. Initial clinical trial data demonstrated that tumors without the ESR1 mutation are less likely to benefit from the SERDs and may still respond to SERMs or AIs, including tumors previously exposed to hormonal therapy. Testing for ESR1 mutation in ongoing clinical trials and in hormonal therapy for breast cancer is highly recommended. Novel protein degradation technologies such as proteolysis-targeting chimera (PROTACS), molecular glue degrader (MGD), and lysosome-targeting chimeras (LYTACS) may result in more efficient ER degradation, while ribonuclease-targeting chimeras (RIBOTAC) and small interfering RNA (siRNA) may inhibit the production of ER protein.
Keywords: Aptamers; Clinical trials; LYTAC; Oral SERDs; PROTC; Protein degradation; RIBOTAC; Selective estrogen receptor degraders.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: A Cancer Journal for Clinicians. 2021;71(1):7–33. - PubMed
-
- Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). . SEER Cancer Statistics Review, 1975–2011, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/archive/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

