Mendelian gene identification through mouse embryo viability screening
- PMID: 36229886
- PMCID: PMC9563108
- DOI: 10.1186/s13073-022-01118-7
Mendelian gene identification through mouse embryo viability screening
Abstract
Background: The diagnostic rate of Mendelian disorders in sequencing studies continues to increase, along with the pace of novel disease gene discovery. However, variant interpretation in novel genes not currently associated with disease is particularly challenging and strategies combining gene functional evidence with approaches that evaluate the phenotypic similarities between patients and model organisms have proven successful. A full spectrum of intolerance to loss-of-function variation has been previously described, providing evidence that gene essentiality should not be considered as a simple and fixed binary property.
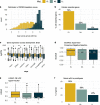
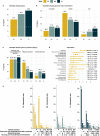
Methods: Here we further dissected this spectrum by assessing the embryonic stage at which homozygous loss-of-function results in lethality in mice from the International Mouse Phenotyping Consortium, classifying the set of lethal genes into one of three windows of lethality: early, mid, or late gestation lethal. We studied the correlation between these windows of lethality and various gene features including expression across development, paralogy and constraint metrics together with human disease phenotypes. We explored a gene similarity approach for novel gene discovery and investigated unsolved cases from the 100,000 Genomes Project.
Results: We found that genes in the early gestation lethal category have distinct characteristics and are enriched for genes linked with recessive forms of inherited metabolic disease. We identified several genes sharing multiple features with known biallelic forms of inborn errors of the metabolism and found signs of enrichment of biallelic predicted pathogenic variants among early gestation lethal genes in patients recruited under this disease category. We highlight two novel gene candidates with phenotypic overlap between the patients and the mouse knockouts.
Conclusions: Information on the developmental period at which embryonic lethality occurs in the knockout mouse may be used for novel disease gene discovery that helps to prioritise variants in unsolved rare disease cases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures