Optimized decision support for selection of transoral robotic surgery or (chemo)radiation therapy based on posttreatment swallowing toxicity
- PMID: 36229990
- PMCID: PMC9972156
- DOI: 10.1002/cam4.5253
Optimized decision support for selection of transoral robotic surgery or (chemo)radiation therapy based on posttreatment swallowing toxicity
Abstract
Background: A primary goal in transoral robotic surgery (TORS) for oropharyngeal squamous cell cancer (OPSCC) survivors is to optimize swallowing function. However, the uncertainty in the outcomes of TORS including postoperative residual positive margin (PM) and extranodal extension (ENE), may necessitate adjuvant therapy, which may cause significant swallowing toxicity to survivors.
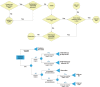
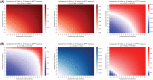
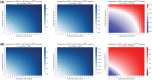
Methods: A secondary analysis was performed on a prospective registry data with low- to intermediate-risk human papillomavirus-related OPSCC possibly resectable by TORS. Decision trees were developed to model the uncertainties in TORS compared with definitive radiation therapy (RT) and chemoradiation therapy (CRT). Swallowing toxicities were measured by Dynamic Imaging Grade of Swallowing Toxicity (DIGEST), MD Anderson Dysphagia Inventory (MDADI), and the MD Anderson Symptom Inventory-Head and Neck (MDASI-HN) instruments. The likelihoods of PM/ENE were varied to determine the thresholds within which each therapy remains optimal.
Results: Compared with RT, TORS resulted in inferior swallowing function for moderate likelihoods of PM/ENE (>60% in short term for all instruments, >75% in long term for DIGEST and MDASI) leaving RT as the optimal treatment. Compared with CRT, TORS remained the optimal therapy based on MDADI and MDASI but showed inferior swallowing outcomes based on DIGEST for moderate-to-high likelihoods of PM/ENE (>75% for short-term and >40% for long-term outcomes).
Conclusion: In the absence of reliable estimation of postoperative PM/ENE concurrent with significant postoperative PM, the overall toxicity level in OPSCC patients undergoing TORS with adjuvant therapy may become more severe compared with patients receiving nonsurgical treatments thus advocating definitive (C)RT protocols.
Keywords: decision analysis; definitive (chemo)radiation therapy; head-and-neck cancer; oropharyngeal squamous cell cancer; posttreatment toxicity; transoral robotic surgery.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Fuller received/receives funding and salary support from directly related to this project from: NIH National Institute of Dental and Craniofacial Research (NIDCR) Academic Industrial Partnership Grant (R01DE028290); NIDCR Establishing Outcome Measures for Clinical Studies of Oral and Craniofacial Diseases and Conditions award (R01DE025248); NIH/NSF NCI Smart Connected Health Program (R01CA257814). Dr. Fuller received/receives funding and salary support from directly unrelated to this project from: NCI Parent Research Project Grant (R01CA258827); NCI Ruth L. Kirschstein NRSA Institutional Research Training Grant (T32CA261856); NIH NIDCR Exploratory/Developmental Research Grant Program (R21DE031082); National Institutes of Health (NIH) National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Program (R01CA214825); NSF/NIH Joint Initiative on Quantitative Approaches to Biomedical Big Data program (R01CA225190); NIH National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Programs for Residents and Clinical Fellows Grant (R25EB025787); NCI Early Phase Clinical Trials in Imaging and Image‐Guided Interventions Program (1R01CA218148); NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672); Small Business Innovation Research Grant Program a sub‐award from Oncospace, Inc. (R43CA254559); The Human BioMolecular Atlas Program (HuBMAP) Integration, Visualization & Engagement (HIVE) Initiative (OT2OD026675) sub‐award; Patient‐Centered Outcomes Research Institute (PCS‐1609‐36,195) sub‐award from Princess Margaret Hospital; National Science Foundation (NSF) Division of Civil, Mechanical, and Manufacturing Innovation (CMMI) grant (NSF 1933369). Dr. Fuller receives grant and infrastructure support from MD Anderson Cancer Center via the Charles and Daneen Stiefel Center for Head and Neck Cancer Oropharyngeal Cancer Research Program; the Program in Image‐guided Cancer Therapy; and the NIH/NCI Cancer Center Support Grant (CCSG) Radiation Oncology and Cancer Imaging Program (P30CA016672). Dr. Fuller has received direct industry grant/in‐kind support, honoraria, and travel funding from Elekta AB. TCS was supported by The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences TL1 Program (TL1 TR003169).
Figures
References
-
- National Cancer Institute – Head and Neck Cancers . Accessed September 10, 2021. https://www.cancer.gov/types/head‐and‐neck/head‐neck‐fact‐sheet
-
- Centers for Disease Control and Prevention – Head and Neck Cancers . Accessed September 29, 2021. https://www.cdc.gov/cancer/headneck/
-
- Jamal Z, Anjum F. Oropharyngeal Squamous Cell Carcinoma. StatPearls; 2020. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

