The PD-1/PD-L1 Pathway: A Perspective on Comparative Immuno-Oncology
- PMID: 36230402
- PMCID: PMC9558501
- DOI: 10.3390/ani12192661
The PD-1/PD-L1 Pathway: A Perspective on Comparative Immuno-Oncology
Abstract
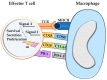
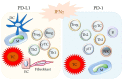
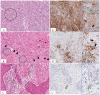
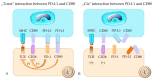
The programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) pathway mainly attracted attention in immuno-oncology, leading to the development of immune checkpoint therapy. It has, however, much broader importance for tissue physiology and pathology. It mediates basic processes of immune tolerance and tissue homeostasis. In addition, it is involved in the pathogenesis of chronic infectious diseases, autoimmunity, and cancer. It is also an important paradigm for comparative pathology as well as the "one health one medicine" concept. The aim of this review is to provide an overview of novel research into the diverse facets of the PD-1/PD-L1 pathway and to give insights into its fine-tuning homeostatic role in a tissue-specific context. This review details early translational research from the discovery phase based on mice as animal models for understanding pathophysiological aspects in human tissues to more recent research extending the investigations to several animal species. The latter has the twofold goal of comparing this pathway between humans and different animal species and translating diagnostic tools and treatment options established for the use in human beings to animals and vice versa.
Keywords: PD-1; PD-L1; cancer; comparative pathology; immuno-oncology; one health one medicine concept; programmed death protein 1; programmed death protein ligand 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

