Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives
- PMID: 36230474
- PMCID: PMC9559463
- DOI: 10.3390/cancers14194547
Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives
Abstract
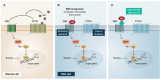
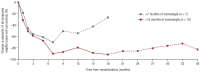
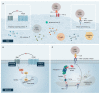
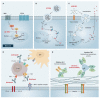
The first-line therapy for locally advanced basal cell carcinoma (laBCC) is Hedgehog pathway inhibitors (HHIs), as they achieve good efficacy and duration of response. However, toxicity in the course of long-term treatment may lead to a decrease in the quality of life, and consequently to interruption or even discontinuation of therapy. As HHI therapy is a balancing act between effectiveness, adverse events, quality of life, and adherence, numerous successful treatment strategies have evolved, such as dose reduction and dose interruptions with on-off treatment schedules or interruptions with re-challenge after progression. As a small percentage of patients show primary or acquired resistance to HHIs, the inhibition of programmed cell death protein 1 (PD-1) has been approved as a second-line therapy, which may also be accompanied by immune-related toxicities and non-response. Thus, optimization of current treatment schedules, novel agents, and combination strategies are urgently needed for laBCC. Here, we narratively model the treatment sequence for patients with laBCC and summarize the current state of approved treatment regimens and therapeutic strategies to optimize the long-term management of laBCC.
Keywords: Hedgehog pathway inhibitors; basal cell carcinoma; immunotherapy; programmed cell death protein 1 inhibitor.
Conflict of interest statement
M.V.H. declares honoraria from MSD, BMS, Roche, Novartis, SUN, Sanofi, Almirall, Biofrontera, and Galderma. C.B. declares honoraria from Almirall, BMS, Immunocore, Leo Pharma, MSD, Novartis, Pierre Fabre, and Sanofi. J.C.H. declares honoraria from Almirall, Amgen, BMS, GSK, MSD, Novartis, Pierre Fabre, Roche, Sanofi, and SUN. All other authors declare no conflicts of interest. R.G. declares research support from Pfizer, Johnson & Johnson, Novartis, SUN, Amgen, Sanofi, Merck Serono, and Kyowa Kirin. Honoraria for lectures: Roche Pharma, Bristol-Myers Squibb, Novartis, MSD, Almirall-Hermal, Amgen, Merck Serono, Bayer, SUN, Pierre Fabre, and Sanofi. Honoraria for advice: Roche Pharma, Bristol-Myers Squibb, Novartis, MSD, Almirall-Hermal, Amgen, Pierre Fabre, Merck Serono, 4SC, SUN, Merck Serono, Sanofi, and Immunocore. Travel support: Pierre Fabre, Roche, Merck Serono, SUN, Bristol-Myers Squibb.
Figures

References
-
- Peris K., Fargnoli M.C., Garbe C., Kaufmann R., Bastholt L., Seguin N.B., Bataille V., Marmol V.D., Dummer R., Harwood C.A., et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

