LiMAx Prior to Radioembolization for Hepatocellular Carcinoma as an Additional Tool for Patient Selection in Patients with Liver Cirrhosis
- PMID: 36230506
- PMCID: PMC9558955
- DOI: 10.3390/cancers14194584
LiMAx Prior to Radioembolization for Hepatocellular Carcinoma as an Additional Tool for Patient Selection in Patients with Liver Cirrhosis
Abstract
Background and aims: Radioembolization (RE) has recently demonstrated a non-inferior survival outcome compared to systemic therapy for advanced hepatocellular carcinoma (HCC). Therefore, current guidelines recommend RE for patients with advanced HCC and preserved liver function who are unsuitable for transarterial chemoembolization (TACE) or systemic therapy. However, despite the excellent safety profile of RE, post-therapeutic hepatic decompensation remains a serious complication that is difficult to predicted by standard laboratory liver function parameters or imaging modalities. LiMAx® is a non-invasive test for liver function assessment, measuring the maximum metabolic capacity for 13C-Methacetin by the liver-specific enzyme CYP 450 1A2. Our study investigates the potential of LiMAx® for predicting post-interventional decompensation of liver function.
Patients and methods: In total, 50 patients with HCC with or without liver cirrhosis and not amenable to TACE or systemic treatments were included in the study. For patients prospectively enrolled in our study, LiMAx® was carried out one day before RE (baseline) and 28 and 90 days after RE. Established liver function parameters were assessed at baseline, day 28, and day 90 after RE. The relationship between baseline LiMAx® and pre-and post-interventional liver function parameters, as well as the ability of LiMAx® to predict hepatic decompensation, were analyzed.
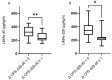
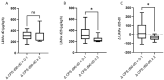
Results: We observed a strong association between baseline LiMAx® and bilirubin, albumin, ALBI grade, and MELD score. Patients presenting with Child-Pugh score B 28 days after RE or with a deterioration in Child-Pugh score by at least one point had a significantly lower baseline LiMAx® compared to those with Child-Pugh score A or with stable Child-Pugh score. The ability of LiMAx® to predict hepatic decompensation after RE was determined using ROC curve analysis and was compared to MELD score and ALBI grade. LiMAx® achieved a substantial AUC of 0.8117, comparable to MELD score and ALBI grade.
Conclusion: Patients with lower LiMAx® values at baseline have a significantly increased risk for hepatic decompensation after RE, despite being categorized as Child-Pugh A. Therefore, LiMAx® can be used as an additional tool to identify patients at high risk of post-interventional hepatic failure.
Keywords: HCC; LiMAx®; enzymatic liver function test; hepatocellular carcinoma; radioembolization; selective internal radiotherapy.
Conflict of interest statement
J.B. received speaker’s fees from BTG and grants from Humedics. G.G. reports grants from Humedics. K.H. reports personal fees from Bayer, personal fees and other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, personal fees from Aktis Oncology, personal fees from Theragnostics, personal fees from Pharma15, personal fees from Debiopharm, personal fees from AstraZeneca, personal fees from Janssen, outside the submitted work. C.M.L. has received advisory and speaker honoraria from Boston, Eisai, Roche, MSD, Falk, Shionogi, Astra Zeneca, C.S.L. Behring, Sobi, and AbbVie. The other authors who have taken part in this study declared that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Figures



References
-
- Vogel A., Martinelli E., Vogel A., Cervantes A., Chau I., Daniele B., Llovet J.M., Meyer T., Nault J.-C., Neumann U., et al. Updated Treatment Recommendations for Hepatocellular Carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021;32:801–805. doi: 10.1016/j.annonc.2021.02.014. - DOI - PubMed
-
- Salem R., Lewandowski R.J., Kulik L., Wang E., Riaz A., Ryu R.K., Sato K.T., Gupta R., Nikolaidis P., Miller F.H., et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

