Targeting Homologous Recombination Deficiency in Ovarian Cancer with PARP Inhibitors: Synthetic Lethal Strategies That Impact Overall Survival
- PMID: 36230543
- PMCID: PMC9563432
- DOI: 10.3390/cancers14194621
Targeting Homologous Recombination Deficiency in Ovarian Cancer with PARP Inhibitors: Synthetic Lethal Strategies That Impact Overall Survival
Abstract
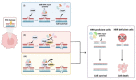
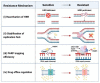
The advent of molecular targeted therapies has made a significant impact on survival of women with ovarian cancer who have defects in homologous recombination repair (HRR). High-grade serous ovarian cancer (HGSOC) is the most common histological subtype of ovarian cancer, with over 50% displaying defective HRR. Poly ADP ribose polymerases (PARPs) are a family of enzymes that catalyse the transfer of ADP-ribose to target proteins, functioning in fundamental cellular processes including transcription, chromatin remodelling and DNA repair. In cells with deficient HRR, PARP inhibitors (PARPis) cause synthetic lethality leading to cell death. Despite the major advances that PARPis have heralded for women with ovarian cancer, questions and challenges remain, including: can the benefits of PARPis be brought to a wider range of women with ovarian cancer; can other drugs in clinical use function in a similar way or with greater efficacy than currently clinically approved PARPis; what can we learn from long-term responders to PARPis; can PARPis sensitise ovarian cancer cells to immunotherapy; and can synthetic lethal strategies be employed more broadly to develop new therapies for women with ovarian cancer. We examine these, and other, questions with focus on improving outcomes for women with ovarian cancer.
Keywords: BRCA; PARP; high-grade serous ovarian cancer; homologous recombination deficiency; homologous recombination repair; niraparib; olaparib; ovarian cancer; rucaparib; synthetic lethal.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Dion L., Carton I., Jaillard S., Nyangoh Timoh K., Henno S., Sardain H., Foucher F., Levêque J., de la Motte Rouge T., Brousse S., et al. The Landscape and Therapeutic Implications of Molecular Profiles in Epithelial Ovarian Cancer. J. Clin. Med. 2020;9:2239. doi: 10.3390/jcm9072239. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

