Novel Insights into Redox-Based Mechanisms for Auranofin-Induced Rapid Cancer Cell Death
- PMID: 36230784
- PMCID: PMC9562029
- DOI: 10.3390/cancers14194864
Novel Insights into Redox-Based Mechanisms for Auranofin-Induced Rapid Cancer Cell Death
Abstract
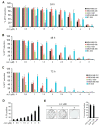
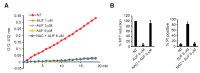
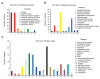
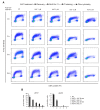
Auranofin (Ridaura®, AUF) is a gold complex originally approved as an antirheumatic agent that has emerged as a potential candidate for multiple repurposed therapies. The best-studied anticancer mechanism of AUF is the inhibition of thioredoxin reductase (TrxR). However, a number of reports indicate a more complex and multifaceted mode of action for AUF that could be cancer cell type- and dose-dependent. In this study, we observed that AUF displayed variable cytotoxicity in five triple-negative breast cancer cell lines. Using representative MDA-MB-231 cells treated with moderate and cytotoxic doses of AUF, we evidenced that an AUF-mediated TrxR inhibition alone may not be sufficient to induce cell death. Cytotoxic doses of AUF elicited rapid and drastic intracellular oxidative stress affecting the mitochondria, cytoplasm and nucleus. A "redoxome" proteomics investigation revealed that a short treatment with a cytotoxic dose AUF altered the redox state of a number of cysteines-containing proteins, pointing out that the cell proliferation/cell division/cell cycle and cell-cell adhesion/cytoskeleton structure were the mostly affected pathways. Experimentally, AUF treatment triggered a dose-dependent S-phase arrest and a rapid disintegration of the actin cytoskeleton structure. Our study shows a new spectrum of AUF-induced early effects and should provide novel insights into the complex redox-based mechanisms of this promising anticancer molecule.
Keywords: auranofin; cancer; oxidative stress; redox regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

