BRAF Inhibitors in Non-Small Cell Lung Cancer
- PMID: 36230797
- PMCID: PMC9562258
- DOI: 10.3390/cancers14194863
BRAF Inhibitors in Non-Small Cell Lung Cancer
Abstract
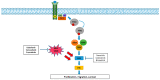
RAF family proteins are serine-threonine kinases that play a central role in the MAPK pathway which is involved in embryogenesis, cell differentiation, cell proliferation and death. Deregulation of this pathway is found in up to 30% of all human cancers and BRAF mutations can be identified in 1.5-3.5% of NSCLC patients. Following the positive results obtained through the combination of BRAF and MEK inhibitors in BRAF-mutant melanoma, the same combination was prospectively assessed in BRAF-mutant NSCLC. In cohort B of the BRF113928 trial, 57 pretreated NSCLC patients were treated with dabrafenib plus trametinib: an ORR of 68.4%, a disease control rate of 80.7%, a median PFS of 10.2 months and a median OS of 18.2 months were observed. Similar results were reported in the first-line setting (cohort C), with an ORR of 63.9%, a DCR of 75% and a median PFS and OS of 10.2 and 17.3 months, respectively. The combination was well tolerated: the main adverse events were pyrexia (64%), nausea (56%), diarrhoea (56%), fatigue (36%), oedema (36%) and vomiting (33%). These positive results led to the approval of the combination of dabrafenib and trametinib for the treatment of BRAF V600E metastatic NSCLC patients regardless of previous therapy. Ongoing research should better define the role of new generation RAF inhibitors for patients with acquired resistance, the activity of chemo-immunotherapy or the combination of TKIs with chemotherapy or with immunotherapy in patients with BRAF-mutated cancers.
Keywords: BRAF; MEK; NSCLC; binimetinib; dabrafenib; encorafenib; trametinib.
Conflict of interest statement
Alessandro Morabito declares the following conflict of interest: speaker’s fee or advisory board: MSD, BMS, Boehringer, Pfizer, Roche, AstraZeneca, Novartis, Takeda, Eli-Lilly. Nicola Normanno declares the following personal financial interests (speaker’s fee and/or advisory boards): MSD, Qiagen, Bayer, Biocartis, Incyte, Roche, BMS, MERCK, Thermofisher, Boehringer Ingelheim, Astrazeneca, Sanofi, Eli Lilly; institutional financial interests (financial support to research projects): MERCK, Sysmex, Thermofisher, QIAGEN, Roche, Astrazeneca, Biocartis. Non-financial interests: President, International Quality Network for Pathology (IQN Path); President, Italian Cancer Society (SIC). All the other authors declare no conflict of interest.
Figures
References
-
- Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., van Schil P.E., Hellmann M.D., et al. ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

