Metabolic Biomarkers Affecting Cell Proliferation and Prognosis in Polycythemia Vera
- PMID: 36230834
- PMCID: PMC9563618
- DOI: 10.3390/cancers14194913
Metabolic Biomarkers Affecting Cell Proliferation and Prognosis in Polycythemia Vera
Abstract
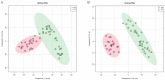
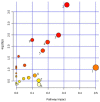
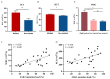
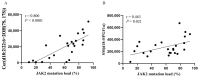
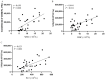
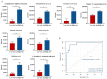
Polycythemia vera (PV) is a malignant clonal hematological disease of hematopoietic stem cells characterized by the proliferation of peripheral blood cells, and JAK2 mutation is one of the main causes of PV peripheral blood cell proliferation. Abnormal cell metabolism is a new feature of malignant proliferation of tumor cells, but the role of metabolism in the pathogenesis and prognosis of PV remains unclear. We analyzed metabolic differences of peripheral blood sera between 32 PV patients and 20 healthy controls (HCs) by liquid chromatography-mass spectrometry (LC-MS) to investigate their relationship with cell proliferation and to screen for prognosis-related metabolic biomarkers. Compared to HC, 33 endogenous metabolites were significantly changed in PV and were involved in fatty acid metabolism, glucose metabolism, sphingolipid metabolism, and amino acid metabolism pathways. Among them, seven metabolites were closely associated with JAK2 mutations, 2 of which may contribute to the proliferation of peripheral blood cells in PV patients. A set of potential prognostic metabolic biomarkers containing four metabolites was identified by a receiver operating characteristic (ROC) curve according to the risk stratification of the PV patients and their combined AUC value of 0.952, with a sensitivity of 90.905% and specificity of 90.909% at the optimal cutoff point. Metabonomics is an important tool for the study of the pathogenesis of PV and the relationship between JAK2 gene mutation. Furthermore, the potential biomarkers of this study may provide a reference for the prognosis of PV.
Keywords: cell proliferation; metabolic biomarkers; metabolomics; polycythemia vera; prognosis.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

Similar articles
-
Diagnostic Performance of Erythropoietin Levels in Polycythemia Vera: Experience at a Comprehensive Cancer Center.Clin Lymphoma Myeloma Leuk. 2021 Apr;21(4):224-229. doi: 10.1016/j.clml.2020.11.002. Epub 2020 Nov 5. Clin Lymphoma Myeloma Leuk. 2021. PMID: 33349602
-
High platelet-to-lymphocyte ratio may differentiate polycythemia vera from secondary polycythemia.Wien Klin Wochenschr. 2022 Jun;134(11-12):483-486. doi: 10.1007/s00508-022-02027-w. Epub 2022 Apr 7. Wien Klin Wochenschr. 2022. PMID: 35391561
-
Current diagnostic criteria for the chronic myeloproliferative disorders (MPD) essential thrombocythemia (ET), polycythemia vera (PV) and chronic idiopathic myelofibrosis (CIMF).Pathol Biol (Paris). 2007 Mar;55(2):92-104. doi: 10.1016/j.patbio.2006.06.002. Epub 2006 Aug 21. Pathol Biol (Paris). 2007. PMID: 16919893 Review.
-
Polycythemia vera: scientific advances and current practice.Semin Hematol. 2005 Oct;42(4):206-20. doi: 10.1053/j.seminhematol.2005.08.003. Semin Hematol. 2005. PMID: 16210034 Review.
-
Polycythemia vera: the current status of preclinical models and therapeutic targets.Expert Opin Ther Targets. 2020 Jul;24(7):615-628. doi: 10.1080/14728222.2020.1762176. Epub 2020 May 18. Expert Opin Ther Targets. 2020. PMID: 32366208 Review.
Cited by
-
Variance quantitative trait loci reveal gene-gene interactions which alter blood traits.medRxiv [Preprint]. 2024 Sep 19:2024.09.18.24313883. doi: 10.1101/2024.09.18.24313883. medRxiv. 2024. PMID: 39371150 Free PMC article. Preprint.
References
-
- Srour S.A., Devesa S.S., Morton L.M., Check D.P., Curtis R.E., Linet M.S., Dores G. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001–2012. Br. J. Haematol. 2016;174:382–396. doi: 10.1111/bjh.14061. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

