CD20 Expression as a Possible Novel Prognostic Marker in CLL: Application of EuroFlow Standardization Technique and Normalization Procedures in Flow Cytometric Expression Analysis
- PMID: 36230840
- PMCID: PMC9562902
- DOI: 10.3390/cancers14194917
CD20 Expression as a Possible Novel Prognostic Marker in CLL: Application of EuroFlow Standardization Technique and Normalization Procedures in Flow Cytometric Expression Analysis
Abstract
Background: CD20 expression is a controversial issue regarding response prediction to anti-CD20 therapy in chronic lymphocytic leukemia (CLL).
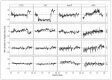
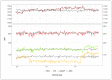
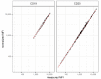
Methods: Median fluorescence intensities (MFIs) of standard fluorescence beads from the daily calibration of flow cytometers according to EuroFlow protocols were used to establish a normalization approach to study CD20 expression on CLL cells. CD20 MFI was retrospectively assessed prior to and during treatment from flow cytometric measurements of peripheral blood in patients with different depths of molecular response in the four phase-II CLL2-BXX trials (BIG; BAG; BIO; BCG; N = 194) administering either Obinutuzumab or Ofatumumab in combination with targeted agents.
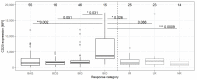
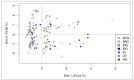
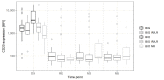
Results: No significant difference was observed between the normalized and measured MFIs of CD19 and CD20 on CLL cells. During treatment, CD20 expression levels on CLL cells did not significantly differ between the four investigated different treatment schemes, but a strong molecular response to Ofatumumab seemed to correlate with higher CD20 expression prior to therapy.
Conclusions: Standardized staining and instrument monitoring enable a robust assessment of longitudinal biological variations of marker expression based on MFI values. Obinutuzumab showed a higher proportion of patients with a strong MRD response independent from initial CD20 expression, whereas high pre-therapeutic CD20 expression levels seem to correlate with a profound response to Ofatumumab.
Keywords: CD20 expression; MRD; flow cytometry normalization.
Conflict of interest statement
A.S., P.J.W., S.K., M.S., A.L., and M.K. declare no conflict of interest. B.E.: Consulting or Advisory Boards for Janssen, Novartis, AbbVie, BMS, ArQule, AstraZeneca, Oxford Biomedica (UK), MSD, Miltenyi, Lilly, Speaker / Speaker’s Bureau: Janssen, Roche, AbbVie, Novartis, Celgene, AstraZeneca, Adaptive Biotechnologies, Hexal; Research funding: Janssen, Gilead, Roche, AbbVie, BeiGene, Astra Zeneca; Travel support: Janssen, Roche, AbbVie. P.C.: research funding by Acerta, AstraZeneca, Beigene, F. Hoffmann-LaRoche, Gilead, Janssen-Cilag, and Novartis, honoraria for scientific talks by AbbVie, AstraZeneca, F. Hoffmann-LaRoche and Janssen-Cilag, advisory boards by AbbVie, AstraZeneca and BeiGene, travel grants by AbbVie, AstraZeneca, F. Hoffmann LaRoche, and Janssen-Cilag. M.H.: Honoraria (speaker’s bureau and/or advisory board) by Roche, Gilead, Janssen, Bristol Myers Squibb, AbbVie, AstraZeneca, Research support by Roche, Gilead, Janssen, Bristol Myers Squibb, AbbVie, AstraZeneca. J.v.T.: advisor or consultant for AbbVie, AstraZeneca, Janssen, and Roche; honoraria from AbbVie, AstraZeneca, Janssen, and Roche, research funding from Janssen and Roche and travel support from AbbVie, AstraZeneca, Janssen, and Roche. K.F.: Honoraria by AbbVie, Roche, Consulting or Advisory Role by AbbVie, AstraZeneca, Other (Travel) by Roche. S.B.: Research funding from AbbVie, Celgene, Janssen, and Roche; consultancy or advisory role for AbbVie, AstraZeneca, Celgene, Roche, Amgen, and Janssen. C.S: Honoraria (Speaker’s bureau) by AstraZeneca and AbbVie. E.T.: Research support by Roche, Abbvie and Gilead and honoraria, advisory board, speakersbureau with Roche, Abbvie, Beigene and AstraZeneca. M.B.: Consultancy or Advisory Boards: Incyte, Amgen; Speaker’s Bureau: Janssen, Amgen; Research funding: Amgen, Roche, Pharma AG; Reference diagnostics: Affimed, Regeneron, Amgen; Travel support: Amgen. M.R.: Consultancy: MSD, Chugai, Abbvie, Roche; Travel support: MSD, Abbvie, Roche, Celgene; Research funding: Abbvie, Roche.
Figures

References
-
- Beers S.A., French R.R., Chan H.T.C., Lim S.H., Jarrett T.C., Vidal R.M., Wijayaweera S.S., Dixon S.V., Kim H., Cox K.L., et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: Implications for antibody selection. Blood. 2010;115:5191–5201. doi: 10.1182/blood-2010-01-263533. - DOI - PubMed
-
- Patz M., Isaeva P., Forcob N., Müller B., Frenzel L.P., Wendtner C.M., Klein C., Umana P., Hallek M., Krause G. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br. J. Haematol. 2011;152:295–306. doi: 10.1111/j.1365-2141.2010.08428.x. - DOI - PubMed
-
- Bologna L., Gotti E., Manganini M., Rambaldi A., Intermesoli T., Introna M., Golay J. Mechanism of Action of Type II, Glycoengineered, Anti-CD20 Monoclonal Antibody GA101 in B-Chronic Lymphocytic Leukemia Whole Blood Assays in Comparison with Rituximab and Alemtuzumab. J. Immunol. 2011;186:3762–3769. doi: 10.4049/jimmunol.1000303. - DOI - PubMed
-
- Bologna L., Gotti E., da Roit F., Intermesoli T., Rambaldi A., Introna M., Golay J. Ofatumumab Is More Efficient than Rituximab in Lysing B Chronic Lymphocytic Leukemia Cells in Whole Blood and in Combination with Chemotherapy. J. Immunol. 2013;190:231–239. doi: 10.4049/jimmunol.1202645. - DOI - PubMed
LinkOut - more resources
Full Text Sources

