Inhibition of Mitochondrial Redox Signaling with MitoQ Prevents Metastasis of Human Pancreatic Cancer in Mice
- PMID: 36230841
- PMCID: PMC9562676
- DOI: 10.3390/cancers14194918
Inhibition of Mitochondrial Redox Signaling with MitoQ Prevents Metastasis of Human Pancreatic Cancer in Mice
Abstract
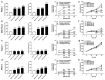
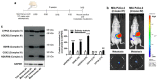
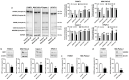
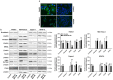
At diagnosis, about 35% of pancreatic cancers are at the locally invasive yet premetastatic stage. Surgical resection is not a treatment option, leaving patients with a largely incurable disease that often evolves to the polymetastatic stage despite chemotherapeutic interventions. In this preclinical study, we hypothesized that pancreatic cancer metastasis can be prevented by inhibiting mitochondrial redox signaling with MitoQ, a mitochondria-targeted antioxidant. Using four different cancer cell lines, we report that, at clinically relevant concentrations (100-500 nM), MitoQ selectively repressed mesenchymal pancreatic cancer cell respiration, which involved the inhibition of the expression of PGC-1α, NRF1 and a reduced expression of electron-transfer-chain complexes I to III. MitoQ consequently decreased the mitochondrial membrane potential and mitochondrial superoxide production by these cells. Phenotypically, MitoQ further inhibited pancreatic cancer cell migration, invasion, clonogenicity and the expression of stem cell markers. It reduced by ~50% the metastatic homing of human MIA PaCa-2 cells in the lungs of mice. We further show that combination treatments with chemotherapy are conceivable. Collectively, this study indicates that the inhibition of mitochondrial redox signaling is a possible therapeutic option to inhibit the metastatic progression of pancreatic cancer.
Keywords: MitoQ; cancer metabolism; cancer metastasis; mitochondria; pancreatic ductal adenocarcinoma (PDAC); reactive oxygen species (ROS); redox signaling.
Conflict of interest statement
T.C. and P.S. are inventors of patent application EP21175397.5 “Molecular signature for assessing the responsiveness of cancer to mitochondria-targeted antioxidants”. P.S., F.D. and F.P.D. are involved in a clinical collaboration with Antipodean Pharmaceuticals Inc. for the prevention of breast cancer metastasis. M.P.M. consults for MitoQ Inc. and holds patents in mitochondria-targeted therapies. Authors declare no other conflict of interest. In particular, Antipodean Pharmaceuticals Inc. and MitoQ Inc., who possess patent rights on the MitoQ molecule, did not fund the study. Neither them nor the funders were involved in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Grants and funding
- WELBIO-CR-2022A-13/Walloon Excellence in Lifesciences and Biotechnology
- 243188 TUMETABO/FP7/2007-2013 ERC Independent Researcher Starting Grant
- No 860245 THERADNET/European Union's Horizon 2020 research innovation program Marie Skłodowska-Curie
- ARC 09/14-020 and 14/19-058/Actions de Recherche Concertées program of the Communauté Française de Belgique
- UP7-03/Interuniversity Attraction Pole (IAP) from the Belgian Science Policy Office (Belspo)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

