Recent Advances in Bacteria-Based Cancer Treatment
- PMID: 36230868
- PMCID: PMC9563255
- DOI: 10.3390/cancers14194945
Recent Advances in Bacteria-Based Cancer Treatment
Abstract
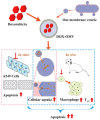
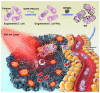
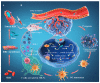
Owing to its unique mechanism of abundant pathogen-associated molecular patterns in antitumor immune responses, bacteria-based cancer immunotherapy has recently attracted wide attention. Compared to traditional cancer treatments such as surgery, chemotherapy, radiotherapy, and phototherapy, bacteria-based cancer immunotherapy exhibits the versatile capabilities for suppressing cancer thanks to its preferentially accumulating and proliferating within tumors. In particular, bacteria have demonstrated their anticancer effect through the toxins, and other active components from the cell membrane, cell wall, and dormant spores. More importantly, the design of engineering bacteria with detoxification and specificity is essential for the efficacy of bacteria-based cancer therapeutics. Meanwhile, bacteria can deliver the cytokines, antibody, and other anticancer theranostic nanoparticles to tumor microenvironments by regulating the expression of the bacterial genes or chemical and physical loading. In this review, we illustrate that naïve bacteria and their components can serve as robust theranostic agents for cancer eradication. In addition, we summarize the recent advances in efficient antitumor treatments by genetically engineering bacteria and bacteria-based nanoparticles. Further, possible future perspectives in bacteria-based cancer immunotherapy are also inspected.
Keywords: bacteria-based cancer treatment; engineered bacteria; tumor therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Bacterial immunotherapy: is it a weapon in our arsenal in the fight against cancer?Front Immunol. 2023 Nov 27;14:1277677. doi: 10.3389/fimmu.2023.1277677. eCollection 2023. Front Immunol. 2023. PMID: 38090593 Free PMC article. Review.
-
Bacteria-cancer interactions: bacteria-based cancer therapy.Exp Mol Med. 2019 Dec 11;51(12):1-15. doi: 10.1038/s12276-019-0297-0. Exp Mol Med. 2019. PMID: 31827064 Free PMC article. Review.
-
Bacteria-Based Cancer Immunotherapy.Adv Sci (Weinh). 2021 Feb 10;8(7):2003572. doi: 10.1002/advs.202003572. eCollection 2021 Apr. Adv Sci (Weinh). 2021. PMID: 33854892 Free PMC article. Review.
-
Bacterial-Mediated Tumor Therapy: Old Treatment in a New Context.Adv Sci (Weinh). 2023 Apr;10(12):e2205641. doi: 10.1002/advs.202205641. Epub 2023 Mar 12. Adv Sci (Weinh). 2023. PMID: 36908053 Free PMC article. Review.
-
Current advances in bacteria-based cancer immunotherapy.Eur J Immunol. 2024 Feb;54(2):e2350778. doi: 10.1002/eji.202350778. Epub 2023 Dec 17. Eur J Immunol. 2024. PMID: 38105295 Review.
Cited by
-
Hacking the Immune Response to Solid Tumors: Harnessing the Anti-Cancer Capacities of Oncolytic Bacteria.Pharmaceutics. 2023 Jul 21;15(7):2004. doi: 10.3390/pharmaceutics15072004. Pharmaceutics. 2023. PMID: 37514190 Free PMC article. Review.
-
The application of bacteria-nanomaterial hybrids in antitumor therapy.J Nanobiotechnology. 2024 Sep 4;22(1):536. doi: 10.1186/s12951-024-02793-x. J Nanobiotechnology. 2024. PMID: 39227831 Free PMC article. Review.
-
Bacterial immunotherapy: is it a weapon in our arsenal in the fight against cancer?Front Immunol. 2023 Nov 27;14:1277677. doi: 10.3389/fimmu.2023.1277677. eCollection 2023. Front Immunol. 2023. PMID: 38090593 Free PMC article. Review.
-
Supercharged precision killers: Genetically engineered biomimetic drugs of screened metalloantibiotics against Acinetobacter baumanni.Sci Adv. 2024 Mar 22;10(12):eadk6331. doi: 10.1126/sciadv.adk6331. Epub 2024 Mar 22. Sci Adv. 2024. PMID: 38517956 Free PMC article.
-
Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach.Int J Mol Sci. 2024 Jan 16;25(2):1110. doi: 10.3390/ijms25021110. Int J Mol Sci. 2024. PMID: 38256183 Free PMC article. Review.
References
-
- Arweiler N.B., Netuschil L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016;902:45–60. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

