TNTdetect.AI: A Deep Learning Model for Automated Detection and Counting of Tunneling Nanotubes in Microscopy Images
- PMID: 36230881
- PMCID: PMC9562025
- DOI: 10.3390/cancers14194958
TNTdetect.AI: A Deep Learning Model for Automated Detection and Counting of Tunneling Nanotubes in Microscopy Images
Abstract
Background: Tunneling nanotubes (TNTs) are cellular structures connecting cell membranes and mediating intercellular communication. TNTs are manually identified and counted by a trained investigator; however, this process is time-intensive. We therefore sought to develop an automated approach for quantitative analysis of TNTs.
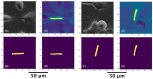
Methods: We used a convolutional neural network (U-Net) deep learning model to segment phase contrast microscopy images of both cancer and non-cancer cells. Our method was composed of preprocessing and model development. We developed a new preprocessing method to label TNTs on a pixel-wise basis. Two sequential models were employed to detect TNTs. First, we identified the regions of images with TNTs by implementing a classification algorithm. Second, we fed parts of the image classified as TNT-containing into a modified U-Net model to estimate TNTs on a pixel-wise basis.
Results: The algorithm detected 49.9% of human expert-identified TNTs, counted TNTs, and calculated the number of TNTs per cell, or TNT-to-cell ratio (TCR); it detected TNTs that were not originally detected by the experts. The model had 0.41 precision, 0.26 recall, and 0.32 f-1 score on a test dataset. The predicted and true TCRs were not significantly different across the training and test datasets (p = 0.78).
Conclusions: Our automated approach labeled and detected TNTs and cells imaged in culture, resulting in comparable TCRs to those determined by human experts. Future studies will aim to improve on the accuracy, precision, and recall of the algorithm.
Keywords: TNT; artificial intelligence; automated cell counting; biomarker; cancer; cells; deep learning; machine learning; microscopy; tunneling nanotubes.
Conflict of interest statement
E.L. reports research grants from the American Association for Cancer Research (AACR-Novocure Tumor-Treating Fields Research Award) and the Minnesota Ovarian Cancer Alliance; honorarium and travel expenses for a research talk at GlaxoSmithKline, 2016; honoraria and travel expenses for lab-based research talks and equipment for laboratory-based research, Novocure, Ltd., 2018-present; honorarium (donated to lab) for panel discussion organized by Antidote Education for a CME module on diagnostics and treatment of HER2+ gastric and colorectal cancers, Daiichi-Sankyo, 2021; consultant, Nomocan Pharmaceuticals (unpaid); Scientific Advisory Board Member, Minnetronix, LLC, 2018-present (unpaid); consultant and speaker honorarium, Boston Scientific US, 2019; institutional Principal Investigator for clinical trials sponsored by Celgene, Novocure, Intima Biosciences, and the National Cancer Institute, and University of Minnesota membership in the Caris Life Sciences Precision Oncology Alliance (unpaid). M.B. is a co-founder, former CEO (2015–2017), and Chief Strategy Officer (2018-present) of Smartlens, Inc.; co-founder and board chair of Magnimind Academy (2016-present); co-founder and advisor of Wowso (2017–2020); co-founder of NanoEye, Inc. (2017-present); co-founder of Nanosight Diagnostic, Inc. (2017-present); and co-founder and CEO of TechDev Academy (2019-present). C.B.P. reports a research grant from the American Association for Cancer Research (AACR-Novocure Career Development Award for Tumor Treating Fields Research), 2022-present; equipment for laboratory-based research, Novocure, Ltd., 2022-present; consultant honoraria and travel expenses, Novocure, Ltd., 2017-present. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Nagaki K., Furuta T., Yamaji N., Kuniyoshi D., Ishihara M., Kishima Y., Murata M., Hoshino A., Takatsuka H. Effectiveness of Create ML in microscopy image classifications: A simple and inexpensive deep learning pipeline for non-data scientists. Chromosome Res. 2021;29:361–371. doi: 10.1007/s10577-021-09676-z. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

