The Nicotinic Receptor Polymorphism rs16969968 Is Associated with Airway Remodeling and Inflammatory Dysregulation in COPD Patients
- PMID: 36230899
- PMCID: PMC9563397
- DOI: 10.3390/cells11192937
The Nicotinic Receptor Polymorphism rs16969968 Is Associated with Airway Remodeling and Inflammatory Dysregulation in COPD Patients
Abstract
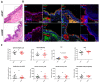
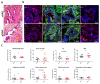
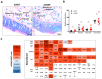
Genome-wide association studies unveiled the associations between the single nucleotide polymorphism rs16969968 of CHRNA5, encoding the nicotinic acetylcholine receptor alpha5 subunit (α5SNP), and nicotine addiction, cancer, and COPD independently. Here, we investigated α5SNP-induced epithelial remodeling and inflammatory response in human COPD airways. We included 26 α5SNP COPD patients and 18 wild-type α5 COPD patients in a multi-modal study. A comparative histologic analysis was performed on formalin-fixed paraffin-embedded lung tissues. Isolated airway epithelial cells from bronchial brushings were cultivated in the air-liquid interface. Broncho-alveolar fluids were collected to detect inflammatory mediators. Ciliogenesis was altered in α5SNP COPD bronchial and bronchiolar epithelia. Goblet cell hyperplasia was exacerbated in α5SNP small airways. The broncho-alveolar fluids of α5SNP COPD patients exhibited an increase in inflammatory mediators. The involvement of the rs16969968 polymorphism in airway epithelial remodeling and related inflammatory response in COPD prompts the development of innovative personalized diagnostic and therapeutic strategies.
Keywords: COPD; airways; epithelial remodeling; inflammation; nicotinic receptors; rs16969968.
Conflict of interest statement
Deslée reports personal fees from Nuvaira, personal fees from BTG/PneumRx, personal fees from Chiesi, personal fees from Boehringer, and personal fees from Astra Zeneca, outside the submitted work. Dormoy reports personal fees from Chiesi and Astra Zeneca outside the submitted work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Lu Z., Van Eeckhoutte H.P., Liu G., Nair P.M., Jones B., Gillis C.M., Nalkurthi B.C., Verhamme F., Buyle-Huybrecht T., Vandenabeele P., et al. Necroptosis Signaling Promotes Inflammation, Airway Remodeling, and Emphysema in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021;204:667–681. doi: 10.1164/rccm.202009-3442OC. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

