Therapeutic Drug-Induced Metabolic Reprogramming in Glioblastoma
- PMID: 36230918
- PMCID: PMC9563867
- DOI: 10.3390/cells11192956
Therapeutic Drug-Induced Metabolic Reprogramming in Glioblastoma
Abstract
Glioblastoma WHO IV (GBM), the most common primary brain tumor in adults, is a heterogenous malignancy that displays a reprogrammed metabolism with various fuel sources at its disposal. Tumor cells primarily appear to consume glucose to entertain their anabolic and catabolic metabolism. While less effective for energy production, aerobic glycolysis (Warburg effect) is an effective means to drive biosynthesis of critical molecules required for relentless growth and resistance to cell death. Targeting the Warburg effect may be an effective venue for cancer treatment. However, past and recent evidence highlight that this approach may be limited in scope because GBM cells possess metabolic plasticity that allows them to harness other substrates, which include but are not limited to, fatty acids, amino acids, lactate, and acetate. Here, we review recent key findings in the literature that highlight that GBM cells substantially reprogram their metabolism upon therapy. These studies suggest that blocking glycolysis will yield a concomitant reactivation of oxidative energy pathways and most dominantly beta-oxidation of fatty acids.
Keywords: TCA cycle; glioblastoma; glycolysis; metabolism; oxidative phosphorylation (OXPHOS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

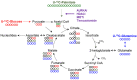
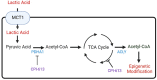
References
-
- Guo G., Gong K., Beckley N., Zhang Y., Yang X., Chkheidze R., Hatanpaa K.J., Garzon-Muvdi T., Koduru P., Nayab A., et al. EGFR ligand shifts the role of EGFR from oncogene to tumour suppressor in EGFR-amplified glioblastoma by suppressing invasion through BIN3 upregulation. Nat. Cell Biol. 2022;24:1291–1305. doi: 10.1038/s41556-022-00962-4. - DOI - PMC - PubMed
-
- Filippo B., Cusimano M., Federico R., Stefano B., Paola M.V.R., Anna R., Stefano C., Barbara C., Peter A., Francesca S., et al. Targeted inducible delivery of immunoactivating cytokines reprograms glioblastoma microenvironment and inhibits growth in mouse models. Sci. Transl. Med. 2022;14:eabl4106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

