Glucose 6-P Dehydrogenase-An Antioxidant Enzyme with Regulatory Functions in Skeletal Muscle during Exercise
- PMID: 36231003
- PMCID: PMC9563910
- DOI: 10.3390/cells11193041
Glucose 6-P Dehydrogenase-An Antioxidant Enzyme with Regulatory Functions in Skeletal Muscle during Exercise
Abstract
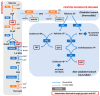
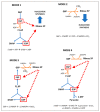
Hypomorphic Glucose 6-P dehydrogenase (G6PD) alleles, which cause G6PD deficiency, affect around one in twenty people worldwide. The high incidence of G6PD deficiency may reflect an evolutionary adaptation to the widespread prevalence of malaria, as G6PD-deficient red blood cells (RBCs) are hostile to the malaria parasites that infect humans. Although medical interest in this enzyme deficiency has been mainly focused on RBCs, more recent evidence suggests that there are broader implications for G6PD deficiency in health, including in skeletal muscle diseases. G6PD catalyzes the rate-limiting step in the pentose phosphate pathway (PPP), which provides the precursors of nucleotide synthesis for DNA replication as well as reduced nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is involved in the detoxification of cellular reactive oxygen species (ROS) and de novo lipid synthesis. An association between increased PPP activity and the stimulation of cell growth has been reported in different tissues including the skeletal muscle, liver, and kidney. PPP activity is increased in skeletal muscle during embryogenesis, denervation, ischemia, mechanical overload, the injection of myonecrotic agents, and physical exercise. In fact, the highest relative increase in the activity of skeletal muscle enzymes after one bout of exhaustive exercise is that of G6PD, suggesting that the activation of the PPP occurs in skeletal muscle to provide substrates for muscle repair. The age-associated loss in muscle mass and strength leads to a decrease in G6PD activity and protein content in skeletal muscle. G6PD overexpression in Drosophila Melanogaster and mice protects against metabolic stress, oxidative damage, and age-associated functional decline, and results in an extended median lifespan. This review discusses whether the well-known positive effects of exercise training in skeletal muscle are mediated through an increase in G6PD.
Keywords: G6PD; NADPH; aging; pentose phosphate pathway; physical training; skeletal muscle.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
-
- Sulek K. Nobel prize in 1931 for Otto Warburg for discovery of the respiratory enzyme. Wiad. Lek. 1968;21:329. - PubMed
-
- Stincone A., Prigione A., Cramer T., Wamelink M.M., Campbell K., Cheung E., Olin-Sandoval V., Grüning N.M., Krüger A., Tauqeer Alam M., et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015;90:927–963. doi: 10.1111/brv.12140. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

