FGFR2 Mutation p.Cys342Arg Enhances Mitochondrial Metabolism-Mediated Osteogenesis via FGF/FGFR-AMPK-Erk1/2 Axis in Crouzon Syndrome
- PMID: 36231091
- PMCID: PMC9563077
- DOI: 10.3390/cells11193129
FGFR2 Mutation p.Cys342Arg Enhances Mitochondrial Metabolism-Mediated Osteogenesis via FGF/FGFR-AMPK-Erk1/2 Axis in Crouzon Syndrome
Abstract
Background: Crouzon syndrome ([OMIM] #123500) caused by FGFR2 mutation is an autosomal dominant syndrome with craniosynostosis, the underlying mechanism of which remains obscure.
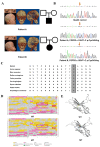
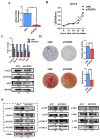
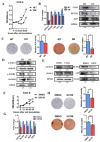
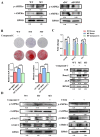
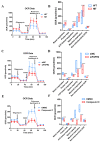
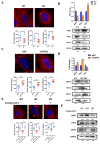
Methods: First, whole exome sequencing was used to screen the possible pathogenic variant in two sporadic patients with Crouzon syndrome. The investigation of primary and secondary structures as well as the conservation analysis of FGFR2 mutation (p.Cys342Arg) was performed. Then, wild-type and mutant overexpression plasmids were constructed and transfected into pre-osteoblastic murine cell line MC3T3-E1 cells. Osteogenesis and mitochondrial metabolism were analyzed by CCK8, ALP staining and ALP activity, alizarin red staining, qRT-PCR, Western blot, seahorse assays and mitochondrial staining. The siRNA targeting FGFR2 and domain negative FGFR2 were designed for verification.
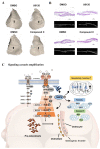
Results: First, FGFR2 mutation (p.Cys342Arg) was detected in two sporadic Chinese Crouzon syndrome patients. FGFR2 p.Cys342Arg promoted the osteogenic differentiation of MC3T3-E1 cells through the upregulation of AMP-activated protein kinase (AMPK)-Erk1/2 signal pathway. Furthermore, FGFR2 p.Cys342Arg enhanced oxidative phosphorylation and converted mitochondrial fusion to the fission of MC3T3-E1, promoting osteogenic differentiation and craniosynostosis in Crouzon syndrome. Additionally, AMPK or Erk1/2 inhibitors delayed the cranial suture closure.
Conclusion: FGFR2 mutation p.Cys342Arg promotes osteogenesis by enhancing mitochondrial metabolism-mediated via FGF/FGFR-AMPK-Erk1/2 axis, which indicates the potential of therapy targeting AMPK or Erk1/2 for syndromic craniosynostosis treatment.
Keywords: AMPK; constitutive activation mutation; craniosynostosis; mitochondrial metabolism; osteogenic differentiation.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures

References
-
- Mitulla B., Hinkel G.K., Lorenz P. [Crouzon syndrome (Mc K 12350)] Kinderarztl. Prax. 1991;59:278–280. - PubMed
-
- Wilkie A.O., Bochukova E.G., Hansen R.M., Taylor I.B., Rannan-Eliya S.V., Byren J.C., Wall S.A., Ramos L., Venancio M., Hurst J.A., et al. Clinical dividends from the molecular genetic diagnosis of craniosynostosis. Am. J. Med. Genet. A. 2007;143A:1941–1949. doi: 10.1002/ajmg.a.31905. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

