Hierarchical Clustering and Trajectory Analyses Reveal Viremia-Independent B-Cell Perturbations in HIV-2 Infection
- PMID: 36231103
- PMCID: PMC9562922
- DOI: 10.3390/cells11193142
Hierarchical Clustering and Trajectory Analyses Reveal Viremia-Independent B-Cell Perturbations in HIV-2 Infection
Abstract
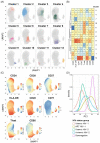
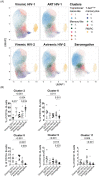
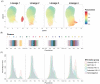
Time to AIDS in HIV-2 infection is approximately twice as long compared to in HIV-1 infection. Despite reduced viremia, HIV-2-infected individuals display signs of chronic immune activation. In HIV-1-infected individuals, B-cell hyperactivation is driven by continuous antigen exposure. However, the contribution of viremia to B-cell perturbations in HIV-2-infected individuals remains largely unexplored. Here, we used polychromatic flow cytometry, consensus hierarchical clustering and pseudotime trajectory inference to characterize B-cells in HIV-1- or HIV-2-infected and in HIV seronegative individuals. We observed increased frequencies of clusters containing hyperactivated T-bethighCD95highCD27int and proliferating T-bet+CD95highCD27+CD71+ memory B-cells in viremic HIV-1 (p < 0.001 and p < 0.001, respectively), viremic HIV-2 (p < 0.001 and p = 0.014, respectively) and in treatment-naïve aviremic HIV-2 (p = 0.004 and p = 0.020, respectively)-infected individuals, compared to seronegative individuals. In contrast, these expansions were not observed in successfully treated HIV-1-infected individuals. Finally, pseudotime trajectory inference showed that T-bet-expressing hyperactivated and proliferating memory B-cell populations were located at the terminal end of two trajectories, in both HIV-1 and HIV-2 infections. As the treatment-naïve aviremic HIV-2-infected individuals, but not the successfully ART-treated HIV-1-infected individuals, showed B-cell perturbations, our data suggest that aviremic HIV-2-infected individuals would also benefit from antiretroviral treatment.
Keywords: B-cell phenotype; CD95; HIV-1; HIV-2; T-bet; immune perturbations; viremia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Esbjornsson J., Mansson F., Kvist A., da Silva Z.J., Andersson S., Fenyo E.M., Isberg P.-E., Biague A.J., Lindman J., Palm A.A., et al. Long-term follow-up of HIV-2-related AIDS and mortality in Guinea-Bissau: A prospective open cohort study. Lancet HIV. 2018;6:e25–e31. doi: 10.1016/S2352-3018(18)30254-6. - DOI - PubMed
-
- van der Loeff M.F., Larke N., Kaye S., Berry N., Ariyoshi K., Alabi A., Tienen C.V., Leligdowicz A., Sarge-Njie R., Siliva Z.D., et al. Undetectable plasma viral load predicts normal survival in HIV-2-infected people in a West African village. Retrovirology. 2010;7:46. doi: 10.1186/1742-4690-7-46. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

