Impact of the COVID-19 Pandemic on the Therapeutic Continuity among Outpatients with Chronic Cardiovascular Therapies
- PMID: 36231403
- PMCID: PMC9566639
- DOI: 10.3390/ijerph191912101
Impact of the COVID-19 Pandemic on the Therapeutic Continuity among Outpatients with Chronic Cardiovascular Therapies
Abstract
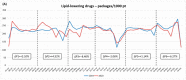
The COVID-19 pandemic poses major challenges to healthcare systems. We aimed to investigate the impact of the pandemic on prescription and adherence patterns of chronic cardiovascular therapies (lipid-lowering [LL], oral antidiabetic drugs [AD], and antihypertensives [AH]) using administrative pharmaceutical databases. For each treatment, two cohorts of prevalent cases in 2019 and 2020 were compared. We evaluated the percentage change in dispensed packages and treatment adherence as a proportion of days covered (PDC). For all therapies, an increase was observed during March-April 2020 (LL: +4.52%; AD: +2.72%; AH: +1.09%), with a sharp decrease in May-June 2020 (LL: -8.40%; AD: -12.09%; AH: -10.54%) compared to 2019. The impact of the COVID-19 pandemic on chronic cardiovascular treatments appears negligible on adherence: 533,414 patients showed high adherence to LL (PDC ≥ 80%) in January-February 2020, and 2.29% became poorly adherent (PDC < 20%) in the following four-month period (vs. 1.98% in 2019). A similar increase was also observed for AH (1.25% with poor adherence in 2020 vs. 0.93% in 2019). For AD, the increase was restrained (1.55% with poor adherence in 2020 vs. 1.37% in 2019). The rush to supply drugs at the beginning of lockdown preserved the continuity of chronic cardiovascular therapies.
Keywords: COVID-19 pandemic; adherence; cardiovascular diseases; chronic treatments; prescriptions.
Conflict of interest statement
All authors declare no support from any organization for the submitted work; no other relationships or activities could appear to have influenced the submitted work. M.C., F.G., M.I., E.O., S.R., M.A., E.T. and E.P. report no disclosures. A.L.C. received research funding and/or honoraria for advisory boards, consultancy, or speaker bureaus from Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Mediolanum, Merck or MSD, Pfizer, Recordati, Rottapharm, Sanofi-Regeneron, and Sigma-Tau. GC received research support from the European Community (EC), the Italian Agency of Drugs (AIFA), and the Italian Ministry of Education, University and Research (MIUR). He took part in a variety of projects that were funded by pharmaceutical companies (i.e., Novartis, GSK, Roche, AMGEN, and BMS). He also received honoraria as a member of the Advisory Board from Roche.
Figures

References
-
- WHO. [(accessed on 20 September 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interact....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

