Multicentric Study to Assess Helicobacter pylori Incidence, Patient Reported Adverse Events, Compliance and Effectiveness, in Real-World Setting
- PMID: 36232149
- PMCID: PMC9566079
- DOI: 10.3390/ijerph191912847
Multicentric Study to Assess Helicobacter pylori Incidence, Patient Reported Adverse Events, Compliance and Effectiveness, in Real-World Setting
Abstract
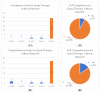
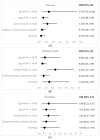
Helicobacter pylori ( H. pylori) plays an important role in chronic gastritis and globally it is estimated to be present in half of the world's population. In Portugal, prevalence reaches 85% and its eradication is recommended using quadruple antibiotic therapy, with or without bismuth. We intended to characterize the prescribed treatments evaluating effectiveness, adverse outcomes and compliance in a real-world setting in a primary care unit. A prospective multicenter observational cohort study was developed in five primary care units of Braga, Portugal. Patients diagnosed with H. pylori infection from August 2021 to January 2022 were included. Data were collected by interview (3 weeks after treatment) and review of medical records. Comparison between two groups of treatment and multivariable analysis was conducted. We estimated 13.4 cases per 1000 adults/year from 185 diagnoses. Therapy with bismuth was the most prescribed (83.8%) with a 96.7% eradication rate. There were no significant differences between treatments. Adverse events were reported in 73.8% of inquiries and female patients were associated with higher reports of nausea (p = 0.03) and metallic taste (p = 0.02). Both eradication schemes were effective and secure. The higher rate of adverse outcomes should be validated but it could influence the debate concerning treating all patients, especially in low gastric cancer-prevalence regions.
Keywords: Helicobacter pylori eradication; adverse events; compliance; effectiveness; quadruple therapy with bismuth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bastos J., Peleteiro B., Barros R., Alves L., Severo M., Pina M.D.F., Pinto H., Carvalho S., Marinho A., Guimarães J.T., et al. Sociodemographic determinants of prevalence and incidence of Helicobacter pylori infection in Portuguese adults. Helicobacter. 2013;18:413–422. doi: 10.1111/hel.12061. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

