Pathological Features in Paediatric Patients with TK2 Deficiency
- PMID: 36232299
- PMCID: PMC9570075
- DOI: 10.3390/ijms231911002
Pathological Features in Paediatric Patients with TK2 Deficiency
Abstract
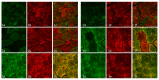
Thymidine kinase (TK2) deficiency causes mitochondrial DNA depletion syndrome. We aimed to report the clinical, biochemical, genetic, histopathological, and ultrastructural features of a cohort of paediatric patients with TK2 deficiency. Mitochondrial DNA was isolated from muscle biopsies to assess depletions and deletions. The TK2 genes were sequenced using Sanger sequencing from genomic DNA. All muscle biopsies presented ragged red fibres (RRFs), and the prevalence was greater in younger ages, along with an increase in succinate dehydrogenase (SDH) activity and cytochrome c oxidase (COX)-negative fibres. An endomysial inflammatory infiltrate was observed in younger patients and was accompanied by an overexpression of major histocompatibility complex type I (MHC I). The immunofluorescence study for complex I and IV showed a greater number of fibres than those that were visualized by COX staining. In the ultrastructural analysis, we found three major types of mitochondrial alterations, consisting of concentrically arranged lamellar cristae, electrodense granules, and intramitochondrial vacuoles. The pathological features in the muscle showed substantial differences in the youngest patients when compared with those that had a later onset of the disease. Additional ultrastructural features are described in the muscle biopsy, such as sarcomeric de-structuration in the youngest patients with a more severe phenotype.
Keywords: TK2 deficiency; mitochondrial myopathies; muscle biopsy; paediatric patients; ragged red fibres; ultrastructural studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

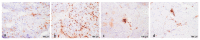

References
-
- Chanprasert S., Wang J., Weng S.W., Enns G.M., Boué D.R., Wong B.L., Mendell J.R., Perry D.A., Sahenk Z., Craigen W.J., et al. Molecular and clinical characterization of the myopathic form of mitochondrial DNA depletion syndrome caused by mutations in the thymidine kinase (TK2) gene. Mol. Genet. Metab. 2013;110:153–161. doi: 10.1016/j.ymgme.2013.07.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

