Alterations of Central Liver Metabolism of Pediatric Patients with Non-Alcoholic Fatty Liver Disease
- PMID: 36232372
- PMCID: PMC9570193
- DOI: 10.3390/ijms231911072
Alterations of Central Liver Metabolism of Pediatric Patients with Non-Alcoholic Fatty Liver Disease
Abstract
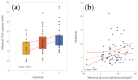
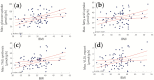
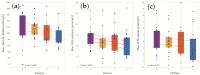
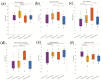
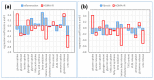
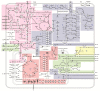
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in children and is associated with overweight and insulin resistance (IR). Almost nothing is known about in vivo alterations of liver metabolism in NAFLD, especially in the early stages of non-alcoholic steatohepatitis (NASH). Here, we used a complex mathematical model of liver metabolism to quantify the central hepatic metabolic functions of 71 children with biopsy-proven NAFLD. For each patient, a personalized model variant was generated based on enzyme abundances determined by mass spectroscopy. Our analysis revealed statistically significant alterations in the hepatic carbohydrate, lipid, and ammonia metabolism, which increased with the degree of obesity and severity of NAFLD. Histologic features of NASH and IR displayed opposing associations with changes in carbohydrate and lipid metabolism but synergistically decreased urea synthesis in favor of the increased release of glutamine, a driver of liver fibrosis. Taken together, our study reveals already significant alterations in the NASH liver of pediatric patients, which, however, are differently modulated by the simultaneous presence of IR.
Keywords: histology; liver tissue; mathematical modeling; plasma profile; proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kohli R., Sunduram S., Mouzaki M., Ali S., Sathya P., Abrams S., Xanthakos S.A., Vos M., Schwimmer J.B. Pediatric Nonalcoholic Fatty Liver Disease: A Report from the Expert Committee on Nonalcoholic Fatty Liver Disease (ECON) J. Pediatr. 2016;172:9–13. doi: 10.1016/j.jpeds.2015.12.016. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

