Anti-TNF Therapies Suppress Adipose Tissue Inflammation in Crohn's Disease
- PMID: 36232469
- PMCID: PMC9570367
- DOI: 10.3390/ijms231911170
Anti-TNF Therapies Suppress Adipose Tissue Inflammation in Crohn's Disease
Abstract
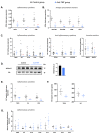
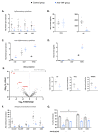
Anti-TNF biologics have been shown to markedly improve the quality of life for patients with Crohn's disease (CD), yet one-third of patients fail to benefit from this treatment. Patients with CD develop a characteristic wrapping of visceral adipose tissue (VAT) in the inflamed intestinal area, termed creeping fat, and it is known that adipose tissue expansion influences the efficacy of anti-TNF drugs. We questioned whether anti-TNF therapies impact the creeping fat in CD, which might affect the outcome of the disease. Adipose tissue biopsies were obtained from a cohort of 14 patients with CD that received anti-TNF drugs and from 29 non-anti-TNF-treated patients (control group) matched by sex, age, and body mass index undergoing surgical interventions for symptomatic complications. We found that anti-TNF therapies restored adipose tissue morphology and suppressed immune cell infiltration in the creeping fat. Additionally, anti-TNF treatments appeared to markedly improve the pro-inflammatory phenotype of adipose-tissue macrophages and adipose-tissue-derived stem cells. Our study provides evidence that anti-TNF medications influence immune cells and progenitor cells in the creeping of patients with CD, suppressing inflammation. We propose that perilesional VAT should be considered when administering anti-TNF therapy in patients with CD.
Keywords: TNF; adalimumab; adipose tissue; creeping fat; infliximab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Li X.-H., Feng S.-T., Cao Q.-H., Coffey J.C., Baker M.E., Huang L., Fang Z.-N., Qiu Y., Lu B.-L., Chen Z.-H., et al. Degree of Creeping Fat Assessed by Computed Tomography Enterography Is Associated with Intestinal Fibrotic Stricture in Patients with Crohn’s Disease: A Potentially Novel Mesenteric Creeping Fat Index. J. Crohns. Colitis. 2021;15:1161–1173. doi: 10.1093/ecco-jcc/jjab005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

