Development of a Novel Primer-TaqMan Probe Set for Diagnosis and Quantification of Meloidogyne enterolobii in Soil Using qPCR and Droplet Digital PCR Assays
- PMID: 36232487
- PMCID: PMC9569833
- DOI: 10.3390/ijms231911185
Development of a Novel Primer-TaqMan Probe Set for Diagnosis and Quantification of Meloidogyne enterolobii in Soil Using qPCR and Droplet Digital PCR Assays
Abstract
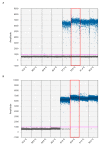
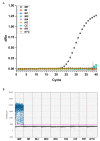
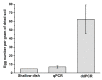
Early detection of pathogens before the planting season is valuable to forecast disease occurrence. Therefore, rapid and reliable diagnostic approaches are urgently needed, especially for one of the most aggressive root knot nematodes, Meloidogyne enterolobii. In this study, we developed a novel primer-TaqMan probe set aimed at M. enterolobii. The primer-probe set was successfully applied in the identification and quantification of M. enterolobii via qPCR technology. It was also suitable for improved PCR technology, known as ddPCR analyses, and this work presents the first application of this technology for plant parasitic nematodes. Compared with qPCR, ddPCR exhibited better performance with regard to analytical sensitivity, which can provide a more accurate detection of M. enterolobii concealed in field soil. In addition, we generated standard curves to calculate the number of eggs in soil using the qPCR and ddPCR platforms. Hopefully, the results herein will be helpful for forecasting disease severity of M. enterolobii infection and adopting effective management strategies.
Keywords: Meloidogyne enterolobii; TaqMan probe; ddPCR; identification; qPCR; quantification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ghareeb R.Y., Hafez E.E., Ibrahim D.S.S. Current management strategies for phytoparasitic nematodes. In: Ansari R.A., Rizvi R., Mahmood I., editors. Management of Phytonematodes: Recent Advances and Future Challenges. Springer; Singapore: 2020. pp. 339–352.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

