O-GlcNAcylation in Renal (Patho)Physiology
- PMID: 36232558
- PMCID: PMC9569498
- DOI: 10.3390/ijms231911260
O-GlcNAcylation in Renal (Patho)Physiology
Abstract
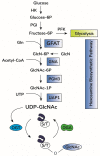
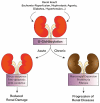
Kidneys maintain internal milieu homeostasis through a well-regulated manipulation of body fluid composition. This task is performed by the correlation between structure and function in the nephron. Kidney diseases are chronic conditions impacting healthcare programs globally, and despite efforts, therapeutic options for its treatment are limited. The development of chronic degenerative diseases is associated with changes in protein O-GlcNAcylation, a post-translation modification involved in the regulation of diverse cell function. O-GlcNAcylation is regulated by the enzymatic balance between O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) which add and remove GlcNAc residues on target proteins, respectively. Furthermore, the hexosamine biosynthetic pathway provides the substrate for protein O-GlcNAcylation. Beyond its physiological role, several reports indicate the participation of protein O-GlcNAcylation in cardiovascular, neurodegenerative, and metabolic diseases. In this review, we discuss the impact of protein O-GlcNAcylation on physiological renal function, disease conditions, and possible future directions in the field.
Keywords: O-GlcNAc; O-GlcNAc transferase; O-GlcNAcase; O-GlcNAcylation; albuminuria; kidney; post-translational modification; renal disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- 308915/2018-6/National Council for Scientific and Technological Development
- 40.1700/2020-8/National Council for Scientific and Technological Development
- 304682/2015-2/National Council for Scientific and Technological Development
- E-26/210.549/2019/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/210.181/2020/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/211.139/2021/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/202.950/2016/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/202.306/2022/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/010.000983/2019/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
LinkOut - more resources
Full Text Sources
Miscellaneous

