Pancreatic Cancer Cells Induce MicroRNA Deregulation in Platelets
- PMID: 36232741
- PMCID: PMC9569638
- DOI: 10.3390/ijms231911438
Pancreatic Cancer Cells Induce MicroRNA Deregulation in Platelets
Abstract
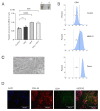
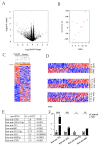
Pancreatic cancer is a pathology with a high mortality rate since it is detected at advanced stages, so the search for early-stage diagnostic biomarkers is essential. Liquid biopsies are currently being explored for this purpose and educated platelets are a good candidate, since they are known to present a bidirectional interaction with tumor cells. In this work, we analyzed the effects of platelets on cancer cells' viability, as determined by MTT, migration using transwell assays, clonogenicity in soft agar and stemness by dilution assays and stem markers' expression. We found that the co-culture of platelets and pancreatic cancer cells increased the proliferation and migration capacity of BXCP3 cells, augmented clonogenicity and induced higher levels of Nanog, Sox2 and Oct4 expression. As platelets can provide horizontal transfer of microRNAs, we also determined the differential expression of miRNAs in platelets obtained from a small cohort of pancreatic cancer patients and healthy subjects. We found clear differences in the expression of several miRNAs between platelets of patients with cancer healthy subjects. Moreover, when we analyzed microRNAs from the platelets of the pancreatic juice and blood derived from each of the cancer patients, interestingly we find differences between the blood- and pancreatic juice-derived platelets suggesting the presence of different subpopulations of platelets in cancer patients, which warrant further analysis.
Keywords: miRNAs; pancreas cancer; tumor-educated platelets.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

