Therapeutic Potential of Mesenchymal Stem Cell-Secreted Factors on Delay in Corneal Wound Healing by Nitrogen Mustard
- PMID: 36232805
- PMCID: PMC9570439
- DOI: 10.3390/ijms231911510
Therapeutic Potential of Mesenchymal Stem Cell-Secreted Factors on Delay in Corneal Wound Healing by Nitrogen Mustard
Abstract
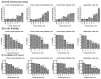
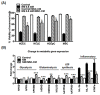
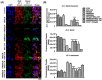
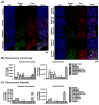
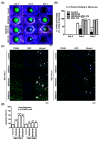
Ocular surface exposure to nitrogen mustard (NM) leads to severe ocular toxicity which includes the separation of epithelial and stromal layers, loss of endothelial cells, cell death, and severe loss of tissue function. No definitive treatment for mustard gas-induced ocular surface disorders is currently available. The research was conducted to investigate the therapeutic potential of mesenchymal stem cell-conditioned media (MSC-CM) in NM-induced corneal wounds. NM was added to different types of corneal cells, the ocular surface of porcine, and the ocular surface of mice, followed by MSC-CM treatment. NM significantly induced apoptotic cell death, cellular ROS (Reactive oxygen species), and reduced cell viability, metabolic gene expression, and mitochondrial function, and, in turn, delayed wound healing. The application of MSC-CM post NM exposure partially restored mitochondrial function and decreased intracellular ROS generation which promoted cell survival. MSC-CM therapy enhanced wound healing process. MSC-CM inhibited NM-induced apoptotic cell death in murine and porcine corneal tissue. The application of MSC-CM following a chemical insult led to significant improvements in the preservation of corneal structure and wound healing. In vitro, ex vivo, and in vivo results suggest that MSC-CM can potentially provide targeted therapy for the treatment of chemical eye injuries, including mustard gas keratopathy (MGK) which presents with significant loss of vision alongside numerous corneal pathologies.
Keywords: apoptotic cell death; cellular ROS; corneal injury; delayed wound healing; mesenchymal stem cell-conditioned media; nitrogen mustard.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures


References
-
- Goswami D.G., Tewari-Singh N., Dhar D., Kumar D., Agarwal C., Ammar D.A., Kant R., Enzenauer R.W., Petrash J.M., Agarwal R. Nitrogen Mustard-Induced Corneal Injury Involves DNA Damage and Pathways Related to Inflammation, Epithelial-Stromal Separation, and Neovascularization. Cornea. 2016;35:257–266. doi: 10.1097/ICO.0000000000000685. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

