Association of Common Variants in OLA1 Gene with Preclinical Atherosclerosis
- PMID: 36232807
- PMCID: PMC9569939
- DOI: 10.3390/ijms231911511
Association of Common Variants in OLA1 Gene with Preclinical Atherosclerosis
Abstract
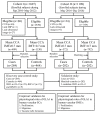
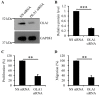
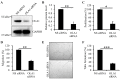
Reactive oxygen species impair the blood vessels, leading to the initiation of atherosclerosis, and migration and proliferation of vascular smooth muscle cells and neovascularization by endothelial cells of vasa vasorum are essential for atherosclerosis development. Obg-like ATPase 1 (OLA1), a negative regulator in cellular responses to oxidative stress, binds to breast cancer susceptibility gene 1 (BRCA1), which protects vascular endothelial and smooth muscle cells against reactive oxygen species. However, it is not known whether OLA1 is genetically correlated with atherosclerosis. Here, we conducted two independent population-based case-control studies to explore the effects of variants in OLA1 genes on preclinical atherosclerosis. A total of 564 and 746 subjects who had thicker and normal carotid intima-media thickness (cIMT), respectively, were enrolled. Among 55 screened SNPs, rs35145102, rs201641962, rs12466587, rs4131583, and rs16862482 in OLA1 showed significant associations with cIMT. SNP rs35145102 is a 3'-utr variant and correlates with the differential expression of OLA1 in immune cells. These five genetic markers form a single closely linked block and H1-ATTGT and H2-GCCTC were the top two most prevalent 5-locus haplotypes. The H1 + H1 genotype negatively and H1 + H2 genotype positively correlated with thicker cIMT. The five identified SNPs in the OLA1 gene showed significant correlations with cIMT. Furthermore, we found that OLA1 was required for migration and proliferation of human aortic endothelial and smooth muscle cells and regulated vascular tube formation by human aortic endothelial cells. Therefore, these genetic variants in the OLA1 gene may serve as markers for risk prediction of atherosclerotic diseases.
Keywords: BRCA1; OLA1; atherosclerosis; carotid intima–media thickness; genetic association study; population-based study; single nucleotide polymorphism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Decreased OLA1 (Obg-Like ATPase-1) Expression Drives Ubiquitin-Proteasome Pathways to Downregulate Mitochondrial SOD2 (Superoxide Dismutase) in Persistent Pulmonary Hypertension of the Newborn.Hypertension. 2019 Oct;74(4):957-966. doi: 10.1161/HYPERTENSIONAHA.119.13430. Epub 2019 Sep 3. Hypertension. 2019. PMID: 31476900 Free PMC article.
-
OLA1, an Obg-like ATPase, suppresses antioxidant response via nontranscriptional mechanisms.Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15356-61. doi: 10.1073/pnas.0907213106. Epub 2009 Aug 24. Proc Natl Acad Sci U S A. 2009. PMID: 19706404 Free PMC article.
-
Common Genetic Variants on Bone Morphogenetic Protein Receptor Type IB (BMPR1B) Gene Are Predictive for Carotid Intima-Media Thickness.Circ J. 2019 Mar 25;83(4):749-756. doi: 10.1253/circj.CJ-18-1046. Epub 2019 Feb 2. Circ J. 2019. PMID: 30713213
-
Associations of Common Genetic Variants on IL-17 Genes and Carotid Intima-Media Thickness.J Atheroscler Thromb. 2018 Nov 1;25(11):1156-1167. doi: 10.5551/jat.44453. Epub 2018 Apr 24. J Atheroscler Thromb. 2018. PMID: 29695654 Free PMC article.
-
The Function of BARD1 in Centrosome Regulation in Cooperation with BRCA1/OLA1/RACK1.Genes (Basel). 2020 Jul 24;11(8):842. doi: 10.3390/genes11080842. Genes (Basel). 2020. PMID: 32722046 Free PMC article. Review.
Cited by
-
Identification and development of Tetra-ARMS PCR-based screening test for a genetic variant of OLA1 (Tyr254Cys) in the human failing heart.medRxiv [Preprint]. 2023 Oct 19:2023.10.16.23296746. doi: 10.1101/2023.10.16.23296746. medRxiv. 2023. Update in: PLoS One. 2024 Jun 18;19(6):e0293105. doi: 10.1371/journal.pone.0293105. PMID: 37905026 Free PMC article. Updated. Preprint.
-
Identification and development of Tetra-ARMS PCR-based screening test for a genetic variant of OLA1 (Tyr254Cys) in the human failing heart.PLoS One. 2024 Jun 18;19(6):e0293105. doi: 10.1371/journal.pone.0293105. eCollection 2024. PLoS One. 2024. PMID: 38889130 Free PMC article.
-
Exosome-mediated effects of BRCA1 on cardiovascular artery disease.Cell Biol Toxicol. 2025 Mar 13;41(1):59. doi: 10.1007/s10565-025-09996-4. Cell Biol Toxicol. 2025. PMID: 40080209 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

