The Translatability of Multiple Sclerosis Animal Models for Biomarkers Discovery and Their Clinical Use
- PMID: 36232832
- PMCID: PMC9570245
- DOI: 10.3390/ijms231911532
The Translatability of Multiple Sclerosis Animal Models for Biomarkers Discovery and Their Clinical Use
Abstract
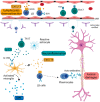
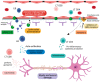
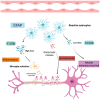
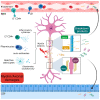
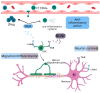
Multiple Sclerosis (MS) is a chronic autoimmune disease affecting the central nervous system which is characterized by demyelinating lesions and axonal damage. MS is a complex disease characterized by important pathophysiological heterogeneity affecting the clinical appearance, progression and therapeutic response for each patient. Therefore, there is a strong unmet need to define specific biomarkers that will reflect the different features of the disease. Experimental autoimmune encephalomyelitis (EAE) is the most commonly used experimental model for the study of MS, as it resembles the pathological features of human MS in many aspects and has allowed for the elucidation of pathogenesis pathways and the validation of certain targets for MS therapies. In this review, we discuss clinically relevant MS molecular biomarkers, divided into five groups based on the key pathological hallmarks of MS: inflammation, blood-brain barrier disruption, myelin and axonal damage, gliosis and, ultimately, repair mechanisms. To address the feasibility of translation between the animal model and human disease, we present an overview of several molecular biomarkers of each category and compare their respective deregulation patterns. We conclude that, like any disease animal model, EAE models can sometimes fail to mimic the entire spectrum of human disease, but they can nonetheless recapitulate the disease's primary hallmarks. We show that the EAE model is a valuable tool for understanding MS physiopathological mechanisms and for identifying biomarkers fundamental for drug development.
Keywords: EAE; animal models; biomarkers; multiple sclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

