Chromone-Containing Allylmorpholines Influence Ion Channels in Lipid Membranes via Dipole Potential and Packing Stress
- PMID: 36232854
- PMCID: PMC9570167
- DOI: 10.3390/ijms231911554
Chromone-Containing Allylmorpholines Influence Ion Channels in Lipid Membranes via Dipole Potential and Packing Stress
Abstract
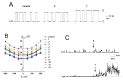
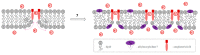
Herein, we report that chromone-containing allylmorpholines can affect ion channels formed by pore-forming antibiotics in model lipid membranes, which correlates with their ability to influence membrane boundary potential and lipid-packing stress. At 100 µg/mL, allylmorpholines 1, 6, 7, and 8 decrease the boundary potential of the bilayers composed of palmitoyloleoylphosphocholine (POPC) by about 100 mV. At the same time, the compounds do not affect the zeta-potential of POPC liposomes, but reduce the membrane dipole potential by 80-120 mV. The allylmorpholine-induced drop in the dipole potential produce 10-30% enhancement in the conductance of gramicidin A channels. Chromone-containing allylmorpholines also affect the thermotropic behavior of dipalmytoylphosphocholine (DPPC), abolishing the pretransition, lowering melting cooperativity, and turning the main phase transition peak into a multicomponent profile. Compounds 4, 6, 7, and 8 are able to decrease DPPC's melting temperature by about 0.5-1.9 °C. Moreover, derivative 7 is shown to increase the temperature of transition of palmitoyloleoylphosphoethanolamine from lamellar to inverted hexagonal phase. The effects on lipid-phase transitions are attributed to the changes in the spontaneous curvature stress. Alterations in lipid packing induced by allylmorpholines are believed to potentiate the pore-forming ability of amphotericin B and gramicidin A by several times.
Keywords: allylmorpholine; chromone; ion channel; membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Local Anesthetics Affect Gramicidin A Channels via Membrane Electrostatic Potentials.J Membr Biol. 2016 Dec;249(6):781-787. doi: 10.1007/s00232-016-9926-x. Epub 2016 Sep 3. J Membr Biol. 2016. PMID: 27592116
-
Differential scanning calorimetric study of the effect of the antimicrobial peptide gramicidin S on the thermotropic phase behavior of phosphatidylcholine, phosphatidylethanolamine and phosphatidylglycerol lipid bilayer membranes.Biochim Biophys Acta. 1999 Mar 4;1417(2):211-23. doi: 10.1016/s0005-2736(99)00004-8. Biochim Biophys Acta. 1999. PMID: 10082797
-
Proton conduction in gramicidin A and in its dioxolane-linked dimer in different lipid bilayers.Biophys J. 1997 Nov;73(5):2489-502. doi: 10.1016/S0006-3495(97)78277-8. Biophys J. 1997. PMID: 9370442 Free PMC article.
-
Transmembrane Signaling with Lipid-Bilayer Assemblies as a Platform for Channel-Based Biosensing.Chem Rec. 2018 Apr;18(4):433-444. doi: 10.1002/tcr.201700046. Epub 2017 Nov 14. Chem Rec. 2018. PMID: 29135061 Review.
-
Temperature-jump and voltage-jump experiments at planar lipid membranes support an aggregational (micellar) model of the gramicidin A ion channel.J Membr Biol. 1986;89(1):23-37. doi: 10.1007/BF01870893. J Membr Biol. 1986. PMID: 2420993 Review.
Cited by
-
Ion and Molecule Transport in Membrane Systems 3.0 and 4.0.Int J Mol Sci. 2023 May 4;24(9):8211. doi: 10.3390/ijms24098211. Int J Mol Sci. 2023. PMID: 37175918 Free PMC article.
References
-
- Manera C., Martinelli A., Nencetti S., Romagnoli F., Rossello A., Giannaccini G., Scatizzi R., Cozzini P., Domiano P. X-ray analysis, theoretical studies and aadrenergic biopharmacological properties of 1-(2,5 dimethoxyphenyl)-2- aminoethanol and its morpholine analogue. Eur. J. Med. Chem. 1994;29:519–525. doi: 10.1016/0223-5234(94)90144-9. - DOI
-
- Bertron J.L., Cho H.P., Garcia-Barrantes P.M., Panarese J.D., Salovich J.M., Nance K.D., Engers D.W., Rook J.M., Blobaum A.L., Niswender C.M., et al. The discovery of VU0486846: Steep SAR from a series of M1 PAMs based on a novel benzomorpholine core. Bioorg. Med. Chem. Lett. 2018;28:2175–2179. doi: 10.1016/j.bmcl.2018.05.009. - DOI - PMC - PubMed
-
- Rook J.M., Bertron J.L., Cho H.P., Garcia-Barrantes P.M., Moran S.P., Maksymetz J.T., Nance K.D., Dickerson J.W., Remke D.H., Chang S., et al. A novel M1 PAM VU0486846 exerts efficacy in cognition models without displaying agonist activity or cholinergic toxicity. ACS Chem. Neurosci. 2018;9:2274–2285. doi: 10.1021/acschemneuro.8b00131. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

