Improved Adipose Tissue Function after Single Anastomosis Duodeno-Ileal Bypass with Sleeve-Gastrectomy (SADI-S) in Diet-Induced Obesity
- PMID: 36232953
- PMCID: PMC9570280
- DOI: 10.3390/ijms231911641
Improved Adipose Tissue Function after Single Anastomosis Duodeno-Ileal Bypass with Sleeve-Gastrectomy (SADI-S) in Diet-Induced Obesity
Abstract
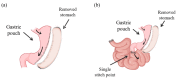
Bariatric surgery has been recognized as the safest and most effective procedure for controlling type 2 diabetes (T2D) and obesity in carefully selected patients. The aim of the present study was to compare the effects of Sleeve Gastrectomy (SG) and Single Anastomosis Duodenoileal Bypass with SG (SADI-S) on the metabolic profile of diet-induced obese rats. A total of 35 four-week-old male Wistar rats were submitted to surgical interventions (sham operation, SG and SADI-S) after 4 months of being fed a high-fat diet. Body weight, metabolic profile and the expression of molecules involved in the control of subcutaneous white (SCWAT), brown (BAT) and beige (BeAT) adipose tissue function were analyzed. SADI-S surgery was associated with significantly decreased amounts of total fat pads (p < 0.001) as well as better control of lipid and glucose metabolism compared to the SG counterparts. An improved expression of molecules involved in fat browning in SCWAT and in the control of BAT and BeAT differentiation and function was observed following SADI-S. Together, our findings provide evidence that the enhanced metabolic improvement and their continued durability after SADI-S compared to SG rely, at least in part, on the improvement of the BeAT phenotype and function.
Keywords: beige adipose tissue; brown adipose tissue; diet-induced obesity; single anastomosis duodenoileal bypass; sleeve gastrectomy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Mingrone G., Panunzi S., De Gaetano A., Guidone C., Iaconelli A., Nanni G., Castagneto M., Bornstein S., Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. - DOI - PubMed
-
- Schauer P.R., Bhatt D.L., Kirwan J.P., Wolski K., Aminian A., Brethauer S.A., Navaneethan S.D., Singh R.P., Pothier C.E., Nissen S.E., et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

