Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture
- PMID: 36232980
- PMCID: PMC9570351
- DOI: 10.3390/ijms231911678
Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture
Abstract
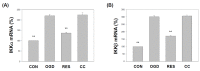
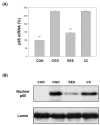
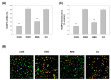
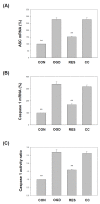
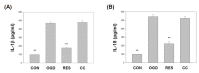
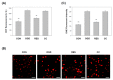
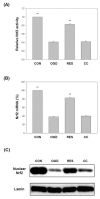
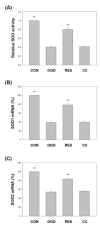
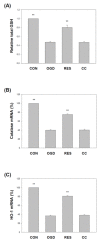
Oxygen glucose deprivation (OGD) can produce hypoxia-induced neurotoxicity and is a mature in vitro model of hypoxic cell damage. Activated AMP-activated protein kinase (AMPK) regulates a downstream pathway that substantially increases bioenergy production, which may be a key player in physiological energy and has also been shown to play a role in regulating neuroprotective processes. Resveratrol is an effective activator of AMPK, indicating that it may have therapeutic potential as a neuroprotective agent. However, the mechanism by which resveratrol achieves these beneficial effects in SH-SY5Y cells exposed to OGD-induced inflammation and oxidative stress in a 3D gelatin scaffold remains unclear. Therefore, in the present study, we investigated the effect of resveratrol in 3D gelatin scaffold cells to understand its neuroprotective effects on NF-κB signaling, NLRP3 inflammasome, and oxidative stress under OGD conditions. Here, we show that resveratrol improves the expression levels of cell viability, inflammatory cytokines (TNF-α, IL-1β, and IL-18), NF-κB signaling, and NLRP3 inflammasome, that OGD increases. In addition, resveratrol rescued oxidative stress, nuclear factor-erythroid 2 related factor 2 (Nrf2), and Nrf2 downstream antioxidant target genes (e.g., SOD, Gpx GSH, catalase, and HO-1). Treatment with resveratrol can significantly normalize OGD-induced changes in SH-SY5Y cell inflammation, oxidative stress, and oxidative defense gene expression; however, these resveratrol protective effects are affected by AMPK antagonists (Compounds C) blocking. These findings improve our understanding of the mechanism of the AMPK-dependent protective effect of resveratrol under 3D OGD-induced inflammation and oxidative stress-mediated cerebral ischemic stroke conditions.
Keywords: 3D scaffold; inflammation; oxidative stress; oxygen-glucose deprivation; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

