Augmented CPT1A Expression Is Associated with Proliferation and Colony Formation during Barrett's Tumorigenesis
- PMID: 36233047
- PMCID: PMC9570428
- DOI: 10.3390/ijms231911745
Augmented CPT1A Expression Is Associated with Proliferation and Colony Formation during Barrett's Tumorigenesis
Abstract
Obesity is a known risk factor for the development of gastroesophageal reflux disease (GERD), Barrett's Esophagus (BE) and the progression to esophageal adenocarcinoma. The mechanisms by which obesity contributes to GERD, BE and its progression are currently not well understood. Recently, changes in lipid metabolism especially in the context of a high fat diet have been linked to GERD and BE leading us to explore whether fatty acid oxidation plays a role in the disease progression from GERD to esophageal adenocarcinoma. To that end, we analyzed the expression of the rate-limiting enzyme, carnitine palmytoyltransferase 1A (CPT1A), in human tissues and cell lines representing different stages in the sequence from normal squamous esophagus to cancer. We determined uptake of palmitic acid, the most abundant fatty acid in human serum, with fluorescent dye-labeled lipids as well as functional consequences of stimulation with palmitic acid relevant to Barrett's tumorigenesis, e.g., proliferation, characteristics of stemness and IL8 mediated inflammatory signaling. We further employed different mouse models including a genetic model of Barrett's esophagus based on IL1β overexpression in the presence and absence of a high fat diet and deoxycholic acid to physiologically mimic gastrointestinal reflux in the mice. Together, our data demonstrate that CPT1A is upregulated in Barrett's tumorigenesis and that experimental palmitic acid is delivered to mitochondria and associated with increased cell proliferation and stem cell marker expression.
Keywords: fatty acid oxidation; gastroesophageal reflux diseases (GERD); high fat diet; inflammation; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
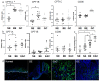

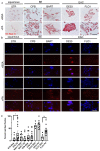
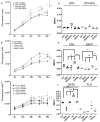
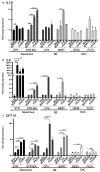

References
-
- El-Serag H.B., Hashmi A., Garcia J., Richardson P., Alsarraj A., Fitzgerald S., Vela M., Shaib Y., Abraham N.S., Velez M., et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: A case-control study. Gut. 2013;63:220–229. doi: 10.1136/gutjnl-2012-304189. - DOI - PMC - PubMed
-
- Hoyo C., Cook M.B., Kamangar F., Freedman N.D., Whiteman D., Bernstein L., Brown L.M., Risch H.A., Ye W., Sharp L., et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: A pooled analysis from the International BEACON Consortium. Int. J. Epidemiol. 2012;41:1706–1718. doi: 10.1093/ije/dys176. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

