Cancer Three-Dimensional Spheroids Mimic In Vivo Tumor Features, Displaying "Inner" Extracellular Vesicles and Vasculogenic Mimicry
- PMID: 36233083
- PMCID: PMC9569704
- DOI: 10.3390/ijms231911782
Cancer Three-Dimensional Spheroids Mimic In Vivo Tumor Features, Displaying "Inner" Extracellular Vesicles and Vasculogenic Mimicry
Abstract
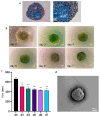
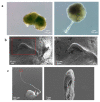
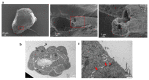
The role of extracellular vesicles (EVs) as mediators of cell-to-cell communication in cancer progression is widely recognized. In vitro studies are routinely performed on 2D culture models, but recent studies suggest that 3D cultures could represent a more valid model. Human ovarian cancer cells CABA I were cultured by the hanging drop method to form tumor spheroids, that were moved to low adhesion supports to observe their morphology by Scanning Electron Microscopy (SEM) and to isolate the EVs. EVs release was verified by SEM and their identity confirmed by morphology (Transmission Electron Microscopy, TEM), size distribution (Nanoparticles Tracking Analysis), and markers (CD63, CD9, TSG-101, Calnexin). CABA I form spheroids with a clinically relevant size, above 400 μm; they release EVs on their external surface and also trap "inner" EVs. They also produce vasculogenic mimicry-like tubules, that bulge from the spheroid and are composed of a hollow lumen delimited by tumor cells. CABA I can be grown as multicellular spheroids to easily isolate EVs. The presence of features typical of in vivo tumors (inner entrapped EVs and vasculogenic mimicry) suggests their use as faithful experimental models to screen therapeutic drugs targeting these pro-tumorigenic processes.
Keywords: extracellular vesicles; ovarian cancer; spheroids; vasculogenic mimicry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

