Nestin+ Mesenchymal Precursors Generate Distinct Spleen Stromal Cell Subsets and Have Immunomodulatory Function
- PMID: 36233119
- PMCID: PMC9569994
- DOI: 10.3390/ijms231911819
Nestin+ Mesenchymal Precursors Generate Distinct Spleen Stromal Cell Subsets and Have Immunomodulatory Function
Abstract
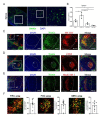
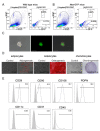
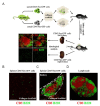
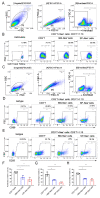
Mesenchymal stromal cells (MSCs) are known to be widespread in many tissues and possess a broad spectrum of immunoregulatory properties. They have been used in the treatment of a variety of inflammatory diseases; however, the therapeutic effects are still inconsistent owing to their heterogeneity. Spleen stromal cells have evolved to regulate the immune response at many levels as they are bathed in a complex inflammatory milieu during infection. Therefore, it is unknown whether they have stronger immunomodulatory effects than their counterparts derived from other tissues. Here, using a transgenic mouse model expressing GFP driven by the Nestin (Nes) promoter, Nes-GFP+ cells from bone marrow and spleen were collected. Artificial lymphoid reconstruction in vivo was performed. Cell phenotype, inhibition of T cell inflammatory cytokines, and in vivo therapeutic effects were assessed. We observed Nes-GFP+ cells colocalized with splenic stromal cells and further demonstrated that these Nes-GFP+ cells had the ability to establish ectopic lymphoid-like structures in vivo. Moreover, we showed that the Nes-GFP+ cells possessed the characteristics of MSCs. Spleen-derived Nes-GFP+ cells exhibited greater immunomodulatory ability in vitro and more remarkable therapeutic efficacy in inflammatory diseases, especially inflammatory bowel disease (IBD) than bone marrow-derived Nes-GFP+ cells. Overall, our data showed that Nes-GFP+ cells contributed to subsets of spleen stromal populations and possessed the biological characteristics of MSCs with a stronger immunoregulatory function and therapeutic potential than bone marrow-derived Nes-GFP+ cells.
Keywords: Nestin; immunoregulation; inflammatory bowel diseases; mesenchymal stromal cells; spleen.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures

Similar articles
-
Nestin+ Peyer's patch resident MSCs enhance healing of inflammatory bowel disease through IL-22-mediated intestinal epithelial repair.Cell Prolif. 2023 Feb;56(2):e13363. doi: 10.1111/cpr.13363. Epub 2022 Nov 20. Cell Prolif. 2023. PMID: 36404603 Free PMC article.
-
Cardiac Nestin+ Mesenchymal Stromal Cells Enhance Healing of Ischemic Heart through Periostin-Mediated M2 Macrophage Polarization.Mol Ther. 2020 Mar 4;28(3):855-873. doi: 10.1016/j.ymthe.2020.01.011. Epub 2020 Jan 15. Mol Ther. 2020. PMID: 31991111 Free PMC article.
-
Nestin-Expressing Precursors Give Rise to Both Endothelial as well as Nonendothelial Lymph Node Stromal Cells.J Immunol. 2016 Oct 1;197(7):2686-94. doi: 10.4049/jimmunol.1501162. Epub 2016 Aug 29. J Immunol. 2016. PMID: 27574301 Free PMC article.
-
Characterization of Nestin, a Selective Marker for Bone Marrow Derived Mesenchymal Stem Cells.Stem Cells Int. 2015;2015:762098. doi: 10.1155/2015/762098. Epub 2015 Jul 6. Stem Cells Int. 2015. PMID: 26236348 Free PMC article. Review.
-
Splenic stromal niches in homeostasis and immunity.Nat Rev Immunol. 2023 Nov;23(11):705-719. doi: 10.1038/s41577-023-00857-x. Epub 2023 Mar 27. Nat Rev Immunol. 2023. PMID: 36973361 Review.
Cited by
-
Promising Markers in the Context of Mesenchymal Stem/Stromal Cells Subpopulations with Unique Properties.Stem Cells Int. 2023 Sep 20;2023:1842958. doi: 10.1155/2023/1842958. eCollection 2023. Stem Cells Int. 2023. PMID: 37771549 Free PMC article. Review.
References
-
- Sacchetti B.F., Michienzi S., di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P.G., Riminucci M., Bianco P. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. - DOI - PubMed
-
- Marrazzo P., Pizzuti V., Zia S., Sargenti A., Gazzola D., Roda B., Bonsi L., Alviano F. Microfluidic Tools for Enhanced Characterization of Therapeutic Stem Cells and Prediction of Their Potential Antimicrobial Secretome. Antibiotics. 2021;10:750. doi: 10.3390/antibiotics10070750. - DOI - PMC - PubMed
-
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

