Systematic Down-Selection of Repurposed Drug Candidates for COVID-19
- PMID: 36233149
- PMCID: PMC9569752
- DOI: 10.3390/ijms231911851
Systematic Down-Selection of Repurposed Drug Candidates for COVID-19
Abstract
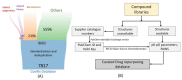
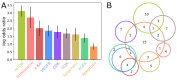
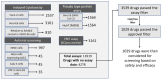
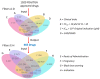
SARS-CoV-2 is the cause of the COVID-19 pandemic which has claimed more than 6.5 million lives worldwide, devastating the economy and overwhelming healthcare systems globally. The development of new drug molecules and vaccines has played a critical role in managing the pandemic; however, new variants of concern still pose a significant threat as the current vaccines cannot prevent all infections. This situation calls for the collaboration of biomedical scientists and healthcare workers across the world. Repurposing approved drugs is an effective way of fast-tracking new treatments for recently emerged diseases. To this end, we have assembled and curated a database consisting of 7817 compounds from the Compounds Australia Open Drug collection. We developed a set of eight filters based on indicators of efficacy and safety that were applied sequentially to down-select drugs that showed promise for drug repurposing efforts against SARS-CoV-2. Considerable effort was made to evaluate approximately 14,000 assay data points for SARS-CoV-2 FDA/TGA-approved drugs and provide an average activity score for 3539 compounds. The filtering process identified 12 FDA-approved molecules with established safety profiles that have plausible mechanisms for treating COVID-19 disease. The methodology developed in our study provides a template for prioritising drug candidates that can be repurposed for the safe, efficacious, and cost-effective treatment of COVID-19, long COVID, or any other future disease. We present our database in an easy-to-use interactive interface (CoviRx that was also developed to enable the scientific community to access to the data of over 7000 potential drugs and to implement alternative prioritisation and down-selection strategies.
Keywords: COVID-19; CoviRx.org; SARS-CoV-2; Variants of Concern (VOC); database; drugs; pandemic; repurposing; therapies; treatments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. [(accessed on 2 February 2022)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- Worldometer. [(accessed on 2 February 2022)]. Available online: https://www.worldometers.info/coronavirus/
-
- The Pandemic’s True Death Toll. [(accessed on 29 September 2022)]. Available online: https://www.economist.com/graphic-detail/coronavirus-excess-deaths-estim....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

