Ribosomal RACK1 Regulates the Dendritic Arborization by Repressing FMRP Activity
- PMID: 36233159
- PMCID: PMC9569565
- DOI: 10.3390/ijms231911857
Ribosomal RACK1 Regulates the Dendritic Arborization by Repressing FMRP Activity
Abstract
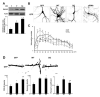
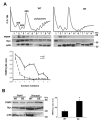
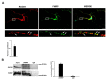
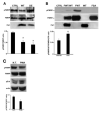
FMRP is an RNA-binding protein that represses the translation of specific mRNAs. In neurons, its depletion determines the exaggerated translation of mRNAs leading to dendritic and axonal aberrant development, two peculiar features of Fragile X syndrome patients. However, how FMRP binds to translational machinery to regulate the translation of its mRNA targets is not yet fully understood. Here, we show that FMRP localizes on translational machinery by interacting with the ribosomal binding protein, Receptor for Activated C Kinase 1 (RACK1). The binding of FMRP to RACK1 removes the translational repressive activity of FMRP and promotes the translation of PSD-95 mRNA, one specific target of FMRP. This binding also results in a reduction in the level of FMRP phosphorylation. We also find that the morphological abnormalities induced by Fmr1 siRNA in cortical neurons are rescued by the overexpression of a mutant form of RACK1 that cannot bind ribosomes. Thus, these results provide a new mechanism underlying FMRP activity that contributes to altered development in FXS. Moreover, these data confirm the role of ribosomal RACK1 as a ribosomal scaffold for RNA binding proteins.
Keywords: FMRP; RACK1; neuroblastoma; neuron differentiation; neurons; ribosomes; translation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures