Promyelocytic Leukemia Protein Potently Restricts Human Cytomegalovirus Infection in Endothelial Cells
- PMID: 36233232
- PMCID: PMC9569661
- DOI: 10.3390/ijms231911931
Promyelocytic Leukemia Protein Potently Restricts Human Cytomegalovirus Infection in Endothelial Cells
Abstract
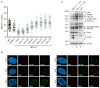
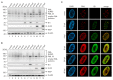
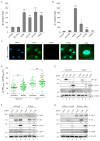
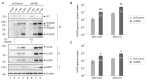
PML nuclear bodies (PML-NBs) are dynamic macromolecular complexes that mediate intrinsic immunity against viruses of different families, including human cytomegalovirus (HCMV). Upon HCMV infection, PML-NBs target viral genomes entering the nucleus and restrict viral immediate-early gene expression by epigenetic silencing. Studies from several groups performed in human fibroblast cells have shown that the major PML-NB components PML, Daxx, Sp100 and ATRX contribute to this repression in a cooperative manner. Their role for HCMV restriction in endothelial cells, however, has not yet been characterized although infected endothelium is thought to play a crucial role for HCMV dissemination and development of vascular disease in vivo. Here, we use conditionally immortalized umbilical vein endothelial cells (HEC-LTT) as a cell culture model to elucidate the impact of PML-NB proteins on lytic HCMV infection. Depletion of individual PML-NB proteins by lentiviral transduction showed a particularly strong antiviral effect of PML in HEC-LTT, compared to human fibroblasts. A closer characterization of this antiviral function revealed that PML may not only effectively inhibit HCMV immediate-early gene expression but also act at later steps of the viral replication cycle. At contrast, we surprisingly noted an antiviral behavior of Daxx in complementary approaches: Depletion of Daxx resulted in decreased viral gene expression, while overexpression of Daxx promoted HCMV infection. In summary, our data demonstrate a cell type-specific effect of PML-NB components on lytic HCMV infection and suggest an important role of PML in the inhibition of HCMV dissemination through infected endothelial cells.
Keywords: Daxx; HCMV; HEC-LTT; PML; PML nuclear bodies; cytomegalovirus; endothelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sinzger C., Digel M., Jahn G. Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 2008;325:63–83. - PubMed
-
- Adler B., Sinzger C. Endothelial cells in human cytomegalovirus infection: One host cell out of many or a crucial target for virus spread? Thromb. Haemost. 2009;102:1057–1063. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

